New antivirals are being sought worldwide to fight back the ever-growing threat of the coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has already claimed more than three million human lives.
Several vaccines have been rolled out in many countries, but it is now clear that the gigantic task of global immunization will not be fulfilled within the current year.
Meanwhile, new variants of the novel coronavirus are constantly emerging across the world. These are often more infectious or virulent than the parent strain. Moreover, they seem to have acquired immune escape capabilities, resisting neutralization by the antibodies elicited by the earlier strains or by vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
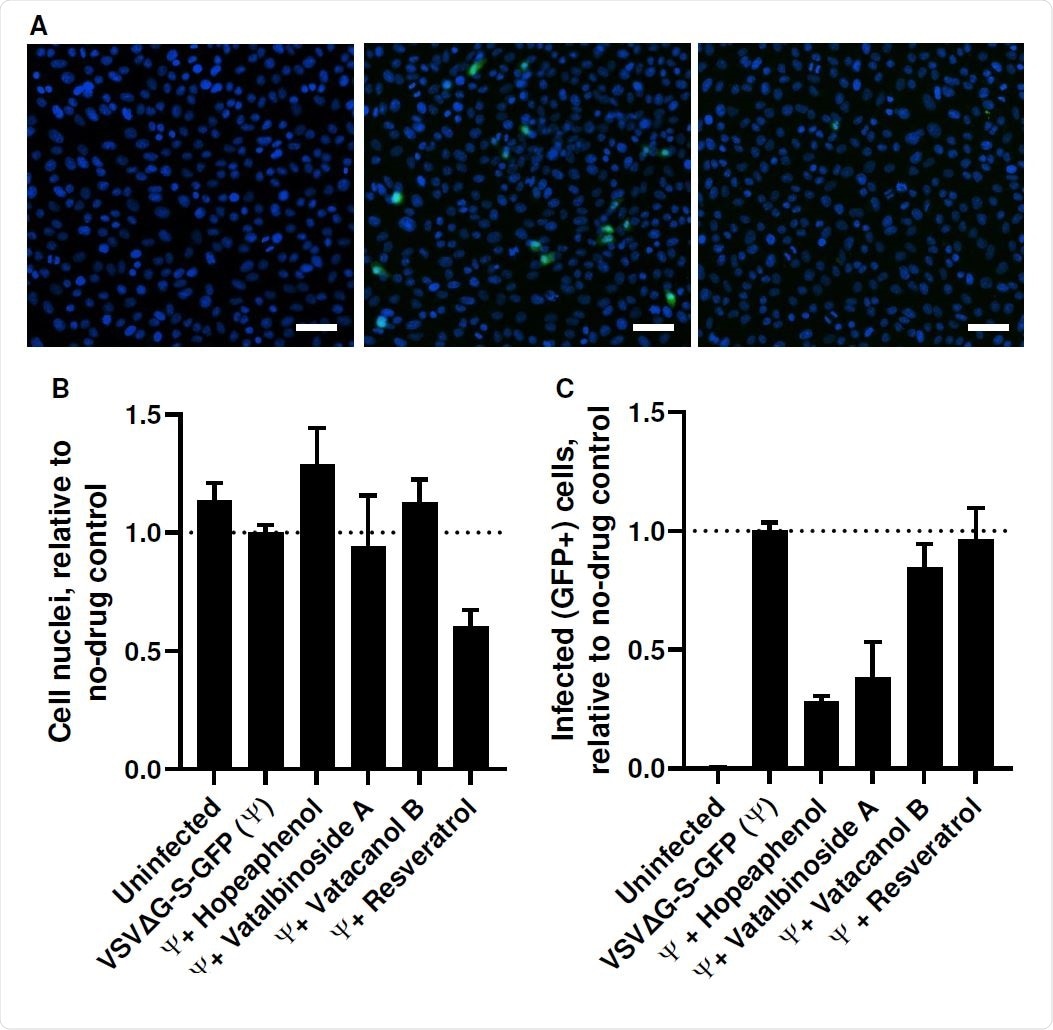
Stilbenoid activity
Many plants including Hopea, Vitis, Shorea and Anisoptera, are sources of stilbenoids such as (–)-hopeaphenol, vatalbinoside A, vaticanol B, and their stereoisomers. Earlier work has shown that, in vitro, these inhibit cell proliferation, inhibit Gram-negative bacteria, fungi and the herpes simplex virus, as well as having anti-inflammatory properties.
In mouse studies, hopeaphenol also normalized triglyceride levels in the plasma following the feeding of olive oil, and glucose levels in mice fed with sugars. Moreover, it prevented liver injury due to the lethal lipopolysaccharides (LPS) that serve as Gram-negative endotoxins.
In these studies, the stilbenoids appeared to be tolerable and safe in vivo, even at high concentrations. These findings indicate the importance of extending these short-term in vivo studies to understand the efficacy of stilbenoids against SARS-CoV-2.
What were the findings?
It is necessary to develop antivirals that are effective against the virus. The current effort began with a library of purified derivatives of natural products.
On analysis, this yielded three compounds of the stilbenoid group. The compound (–)-hopeaphenol is treated as representative of all three.
The researchers found that hopeaphenol prevented the viral spike receptor-binding domain (RBD), expressed on pseudoviruses, from binding to its host cell receptor, the angiotensin-converting enzyme 2 (ACE2). It also prevents the replication of the virus in vitro without affecting the viability of the host cell.
Hopeaphenol continued to be effective against two of the SARS-CoV-2 variants of concern (VOCs), the UK and South African, despite the presence of the mutations that enable them to escape immune neutralization and to be more infectious than the Wuhan or D614G strain.
Thus, these stilbenoid analogs, especially hopeaphenol, offer hope for the development of a broad-spectrum SARS-CoV-2 antiviral, inhibiting entry into host cells. These could be used alone or in combination with antivirals already in use against other viral targets.
More recent molecular docking analysis has shown the potential of stilbenoids to prevent RBD-ACE2 binding. The compound kobophenol A, also a stilbenoid, has also been shown to prevent this interaction at a low inhibitory concentration (50% of binding inhibited at 1.8 μM.
Kobophenol A also inhibited the replication of SARS-CoV-2, with 50% inhibition being observed at 71.6 μM. The current findings with hopeaphenol confirm these earlier results.
Effects of stilbenoids on inhibition of SARS-CoV-2 Mpro activity. A, Demonstration of recombinant Mpro enzymatic activity on a FRET-based fluorogenic peptide substrate. B, Dose response curves of stilbenoids and control inhibitor GC-376 on Mpro enzymatic activity.
However, the researchers failed to observe similar antiviral activity with resveratrol, even at 100 μM, despite earlier reports of 50% effectiveness in preventing the infection of cells in culture by the virus at a tenth of this concentration. One likely reason for this discrepancy could be the fact that the previous work used polymerase chain reaction of the supernatant to check for cell infection, while the current work relied on the observation of cytopathic effects (CPE) caused by the virus within infected cell cultures.
What are the conclusions?
The three compounds studied here showed selective activity against RBD-ACE2 binding relative to another ligand-receptor pair with unrelated physiological activity. However, they must be studied in greater detail to exclude cytotoxicity.
Further research may detect or synthesize analogs with improved safety and therapeutic margins.
Stilbenoids are also easily degraded by oxidation, light and changes in the pH. This vulnerability may explain why vaticanol B showed three-fold lower effectiveness than the other two compounds (hopeaphenol and vatalbinoside A) in preventing RBD-ACE2 binding in vivo, both during pseudovirus inhibition studies and in CPE assays. However, it had the greatest potency in vitro.
The scientists did not observe significant inhibitory activity against the SARS-CoV-2 main protease, however, as seen in a virtual screening study recently. This indicates that the antiviral activity observed here is likely not due to Mpro inhibition.
This is supported by the finding of higher activity against the South African spike variant, compared to both the Wuhan and the UK variant.
However, it may be possible to identify stilbenoid derivatives that are active against both the spike entry protein and the Mpro enzyme, thus delivering a double hit to the virus. This would not just improve the efficacy but reduce the chances of emerging resistance.
These results suggest that spike mutations that promote vaccine-induced viral escape may be distinct from those that might arise from ongoing treatment with hopeaphenol and potentially other stilbenoid-based entry inhibitors.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Tietjen, I. et al. (2021). The natural stilbenoid (–)-hopeaphenol inhibits cellular entry of SARS-CoV-2 USA-WA1/2020, 2 B.1.1.7 and B.1.351 variants. bioRxiv preprint. doi: https://doi.org/10.1101/2021.04.29.442010, https://www.biorxiv.org/content/10.1101/2021.04.29.442010v1
- Peer reviewed and published scientific report.
Tietjen, Ian, Joel Cassel, Emery T. Register, Xiang Yang Zhou, Troy E. Messick, Frederick Keeney, Lily D. Lu, et al. 2021. “The Natural Stilbenoid (–)-Hopeaphenol Inhibits Cellular Entry of SARS-CoV-2 USA-WA1/2020, B.1.1.7, and B.1.351 Variants.” Antimicrobial Agents and Chemotherapy 65 (12). https://doi.org/10.1128/aac.00772-21. https://journals.asm.org/doi/10.1128/AAC.00772-21.