Maintaining the required solubility, stability, efficacy and potency of an engineered biotherapeutic protein is critical after its expression in a recombinant system. Analysis and optimization of the characteristics of biotheraputic proteins such as monoclonal antibodies are performed during the engineering process:
- Potency is improved.
- Solubility and thermostability are improved.
- Downstream process recovery is optimized.
- Potential chemical modification sites are reduced or eliminated.
- Aggregation during storage is reduced.
Directed evolution technology is used to perform protein engineering at Applied Molecular Evolution (AME) (Figure 1), allowing multiple protein properties to be optimized simultaneously. More complex problems can be solved using the screening method over the conventional selection technique, and as a result, more structural and functional data can be obtained. Differential scanning calorimetry (DSC) is one of the analytical and physicochemical techniques used during protein engineering at AME for comparison of thermostability between the parent and engineered antibodies. A protein’s thermal and conformational stability is generally assessed using DSC.
AME approach to directed evolution for protein engineering
A DSC profile provides the melting temperature of proteins or individual domains. It is possible to determine the thermodynamic parameters of the unfolding in the case of a reversible reaction. If the transition temperature (Tm) of a protein is higher, then the protein exhibits better thermostability. Higher Tm or better thermostability is correlated with the reduced aggregation based on the comparison of thermostability data against the data of bioanalytical techniques such as size exclusion chromatography (SEC). Better long-term stability can be expected from antibodies having a higher Tm with reduced tendency towards aggregation, making them a potential candidate to produce better biotherapeutics.
This article discusses the comparison of stability results of DSC and SEC-HPLC methods for three different engineered antibodies. For the antibodies of interest, decreased thermostabilty as determined by DSC is correlated with the higher tendency towards the formation of aggregates during accelerated stability analyses, thereby demonstrating the effectiveness of the DSC method in stability screening of engineered proteins.
Materials and Methods
The experiment involved dialyzing antibody samples against 10mM citrate buffer at a pH level of 6.5 and diluting them to 1mg/mL. An automated MicroCal VPCapillary DSC consisting of a 96-well plate autosampler was used to perform the scans at 200°C/h for a temperature range of 20-95°C, followed by analyzing the scans with Origin 7.0. The non-two-state unfolding model was then used to fit the unfolding transitions of each antibody.
The accelerated stability study involved storing the same set of antibody samples for five days at 60°C and withdrawing aliquots every day for centrifugation at 15,000g for 20 minutes to extract precipitated antibodies. The concentration of soluble proteins and the percentage of soluble high molecular weight aggregates were determined using the supernatant. A Tosoh G3000SWXL (300 X 4.6 mm) column was used to perform analytical SEC at 0.5mL/min flow rate.
Case Study: Characterization of Parent and Engineered Antibodies by DSC
Biological and functional properties of antibodies are well documented, but very little information is available pertaining to the stability and folding of most of the Ig-Fold domains of antibodies in terms of the full-length molecule. Multi-domain folding thermodynamics can be analyzed using DSC to help antibody design. The parent antibody used in this study for engineering had four different unfolding domains as shown by the DSC thermogram (Figure 2A).
Transition temperature can be measured for each unfolding domain: Tm1 to Tm4. The thermostability for the proteins of interest was assessed by monitoring Tm1, which was roughly 69°C for the parent protein (Figure 2A). This study involved engineering the antibodies for optimized potency. Antibody 1 was selected subsequent to the first round of screening for characterizing its biophysical properties. Antibody 1 exhibited a lower Tm1 value (62°C) than the parent antibody as determined by DSC, and therefore, its thermostability was also relatively lower than the parent protein (Figure 2B).
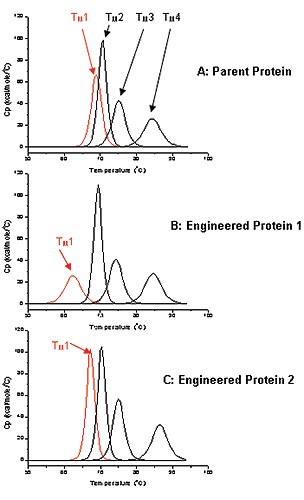
Figure 2. Deconvoluted DSC thermograms for A: Parent Antibody, B: Engineered Antibody 1, C: Engineered Antibody 2. First transition (Tm1) for each antibody is in red, the other 3 transitions are black.
Antibody 2 was obtained from a second round of engineering and screening, showing a Tm value (69°C) equivalent to that of the parent protein (Figure 2C). Therefore, it is expected to have thermostability comparable to that of the parent protein. With greater thermostability, proteins may exhibit higher stability under accelerated stability conditions. This, in turn, results in a biotherapeutic with a longer shelf life. The stability of the parent and engineered antibodies was comparable at 37°C, and therefore, the accelerated stability studies were performed at a temperature close to the Tm1 of Antibody 1 (60°C).
The percentage of soluble protein recovery after storing at 60°C for the designated number of days is depicted in Figure 3, showing higher stability and comparable protein recovery for both parent and Antibody 2 at 60°C. However, the percentage recovery was considerably dropped for Antibody 1 after being stored for five days at 60°C. Correlation is excellent between the stability results determined by DSC and the percent recovery measurements of soluble proteins obtained from the accelerated stability studies for the antibodies studied. However, DSC can assess the stability data more rapidly as it forces the unfolding events.
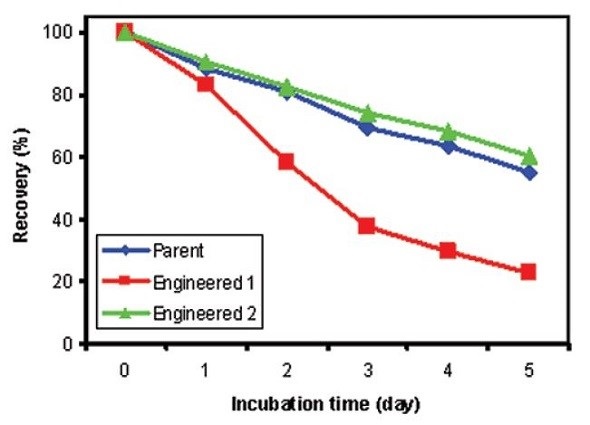
Figure 3. Percent soluble protein after accelerated stability studies for 0, 1, 2, 3, 4, and 5 days at 60°C. Blue: Parent Antibody; Red: Engineered Antibody 1; Green: Engineered Antibody 2.
The soluble aggregate formation was in low amounts but comparable for both parent and Antibody 2 as per the SEC-HPLC results of the accelerated stability studies carried out at 60°C (Figure 4A and 4C). However, the soluble aggregate formation was much higher in the case of Antibody 1 than the parent and engineered antibody 2 (Figure 4B). For the antibodies analyzed, DSC data showed excel correlation with the values determined by longer-term SEC-HPLC aggregation studies. Therefore, protein stability in solution can be rapidly screened using microcalorimetry.
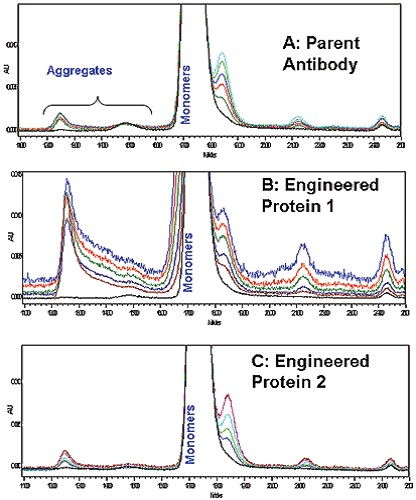
Figure 4. Overlaid SEC-HPLC chromatograms A: Parent Antibody, B; Engineered Antibody 1; C: Engineered Antibody 2.
The potency of Antibody 1 is more than the parent antibody but it has higher amounts of soluble and insoluble aggregation formation than the parent and Antibody 2, according to the accelerated stability studies. This suggests the chance for long-term stability and shelf life problems. However, the improved stability and higher Tm of Antibody 2 in accelerated studies make it a better option for use as a biotherapeutic. However, more research is required for correlating improved stability in accelerated stability analyses and longer shelf life and improved long-term stability of this engineered antibody.
Conclusion
The thermostability of engineered antibodies for improved potency was analyzed using DSC. The decreased thermostability as determined by DSC correlated well with increased aggregate formation during the course of the accelerated stability analyses at 60°C. The stability predictions by DSC was also showed excellent correlation with the SEC-HPLC stability data, suggesting a correlation between the thermostability determined by DSC and the protein stability. Since the DSC data helps identify the likelihood of long-term stability problems, the DSC method is a preferred method to screen and select appropriate engineered proteins.
Acknowledgements
Produced from content authored by Chul-Ho Park, Ph.D. President & CEO; MabPrex, Inc. 6727 Flanders Dr. Suite 212; San Diego, CA 92121; [email protected]
About Malvern Panalytical

Malvern Panalytical provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems.
These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries.
Used at all stages of research, development and manufacturing, Malvern Panalytical’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.