Under the right physiological conditions for gain of function, unfolded proteins can fold into native structures that are thermodynamically-favorable, as illustrated in Figure 1 [1-4].
Native structures are stabilized and solubility is ensured as a result of embedded hydrophobic regions within the protein's interior and also the fact that hydrophilic residues are exposed to aqueous solutions.
Active native proteins use the strongly regulated intermolecular interactions with their binding associates to execute their biological functions under thermodynamic control.
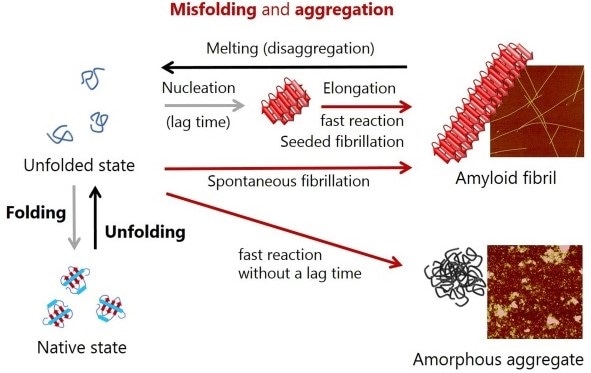
Figure 1. Schematic representation of conformational conversions by protein folding and protein misfolding-induced aggregation. Atomic force microscopy images of amyloid fibrils and amorphous aggregates are shown on the right.
Isothermal titration calorimetry, or ITC, is a powerful technique used for thermodynamic analysis of interactions that occur between inorganic compounds and different types of biomolecules [5-11]. This technique affords thermodynamic parameters for various intermolecular interactions, such as:
- the affinity constant (i.e. dissociation constant) (KD)
- changes in Gibbs free energy (ΔG)
- heat capacity (ΔCp)
- entropy (ΔS)
- enthalpy (ΔH)
- binding stoichiometry (n)
The ITC instrument includes a number of important functions that further expand the utility of the ITC technique to a wide range of binding systems.
These functions include the controlled sample titration in the ITC syringe (such as small molecules) into another sample within the ITC cell (such as macromolecules) and stirring, which makes sure that the ITC cell solution is suitably suspended and combined.
Usually, exogenous stress factors tend to deactivate native proteins, changing thermodynamically stable states from native to globally or locally unfolded states upon exposure of regions that are prone to aggregation.
This successively results in misfolding-coupled irreversible protein aggregation, that is, loss of function. In living biological systems, 'quality control' plays a very important role in the regulation of homeostasis by reducing the aggregation and deactivation of proteins as well as increasing the turnover of proteins [12].
In the pathogenesis of various medical conditions, including neurodegenerative diseases such as Parkinson’s diseases, Alzheimer’s, and amyloidosis, a failure to inhibit aggregation and remove aggregates has been heavily implicated [13,14].
There is a direct association between protein misfolding and diseases and aggregation, and this has actively promoted their analysis. Also, these findings indicate that protein folding, misfolding, function, and disease and aggregation are closely associated and are not separate events. As a result, it is important to have a comprehensive understanding of all the processes and the interactions between them.
Insoluble protein aggregates are divided into amorphous aggregates and amyloid fibrils [15-18]. After a time delay, the latter grows quickly, enabling the formation of productive nuclei through soluble precursor monomers - a nucleation-growth mechanism of the formation of amyloids, that is, spontaneous amyloid fibrillation, as shown in Figure 1 [13-18].
The nucleation step is omitted when amyloid fibrils are added to monomer solution, therefore, the only thing that is observed is the elongation of amyloid fibrils, or in other words, seeded amyloid fibrillation.
Amyloid fibrils are protein aggregates that have a ß structure, morphologically straight, and measure several nm in diameter and several mm long [13,14]. In the majority of cases, amorphous aggregation takes place very quickly even without a substantial lag time, as illustrated in Figure 1 [15-18].
These aggregates are typically rounded, but lack a specific appearance. Curved flexible protofibrils and ß structure-containing oligomers are other types of protein aggregates that have been reported, yet these mostly share the properties of amorphous aggregates and amyloid fibrils.
The past 20 years have witnessed major developments in our understanding of protein folding and the stable nature of globular proteins due to widespread studies using different biophysical methods and theories [1-4,9,19-22].
Calorimetry, especially differential scanning calorimetry (DSC), has been shown to be quite useful for investigating the thermodynamic behavior of protein stability and (un)folding. Many number of DSC studies have demonstrated the thermodynamic nature of thermal unfolding, that is, heat and cold denaturation, by generating a sequence of thermodynamic parameters such as ΔH, ΔG, ΔCp, ΔS, and the midpoint of unfolding temperature (TM) [3,9,19-22].
Irrespective of the type of protein, the signs of these thermodynamic parameters for thermal unfolding — that is negative values of ΔH and ΔG and positive values of ΔCp and ΔS — are the same. Yet, the value of each single parameter varies from one protein to another.
In addition, stability curves for different proteins over temperature, built using the Gibbs-Helmholtz equation and thermodynamic parameters, elucidate how protein stability relies on pressure, mutations, and solvent conditions. As a result, the DSC approach has also been effectively used in antibody drug development.
While DSC has provided a better insight into the thermodynamic behavior of protein folding, only limited knowledge is available about the molecular mechanisms and thermodynamic properties that lead to misfolding and aggregation of proteins.
This is because of technical complexities related to the heterogeneity of aggregates and large size. Moreover, calorimetric methods have not been applied to study the thermodynamics of protein aggregation.
This article briefly shows how calorimetry (ITC and DSC) are uniquely applied to study the misfolding and aggregation of proteins. Observations of seeded and spontaneous formation of amyloids, amyloid fibril associations, dissociation of aggregates, and amorphous aggregation using calorimetry have been explained.
The article also discusses calorimetry-based thermodynamic analyses of protein aggregation and deals with structural features to define protein aggregates using the aggregation’s thermodynamic parameters.
These ground-breaking attempts are anticipated to encourage calorimetry-based thermodynamic analyses of misfolding and aggregation of proteins and may provide a platform for developing treatments and preventing diseases related to aggregation.
Study of protein aggregation using DSC
DSC techniques have been widely employed in thermodynamic investigations of the stability as well as thermal unfolding mechanisms of natively-folded states of proteins, depending on well-defined physicochemical relationships and characteristic transition peaks.
When the occurrence of protein aggregates promotes extensive distortions in the Cp curve and causes irreversible unfolding, thermodynamic analysis of thermal unfolding followed by aggregation is not always possible.
Yet, modifications and deformation of Cp traces and DSC peaks can be used as indicators of aggregate formation, as shown in Figure 2. These are especially useful for confirming the homogeneity of sample solutions, the present molecular species, and for explaining the mechanisms involved in protein (un)folding.
In the year 1998, Litvinovich et al. reported that successive DSC scanning of the recombinant module III-9 from murine fibronectin or rmIII-9 at neutral pH showed the formation of aggregates and multimers of rmIII-9 with a deformed Cp curve and a widened DSC peak with exothermic responses following the thermal transition, as illustrated in Figure 2A [23].
![Thermal unfolding and subsequent aggregation of proteins. Panes A and B: Illustration of DSC thermograms of native folding and aggregation. Pane A: The continuous line represents a Cp curve of rmIII-9 observed and the dotted line indicates a best fit of the peak to a single two-state thermal transition (see Figure 5B of Litvinovich et al. [23] for more details). Pane B: First and second heating of ARQ are shown with continuous and dotted lines, respectively (see Figures 3C and 4D of Rezaei et al. for ARQ [25] for more details). Pane C: DSC thermograms of FNR. First and second heat scans are shown with continuous and dotted lines, respectively. Pane D: Top views of test tubes containing FNR sample solutions before (upper) and after (lower) DSC measurements on the right. Light scattering intensities of 25 mM sodium phosphate buffer (pH 7.4) alone, and FNR samples before and after DSC measurements on the left.](https://www.news-medical.net/image-handler/picture/2017/7/2_Malvern.jpg)
Figure 2. Thermal unfolding and subsequent aggregation of proteins. Panes A and B: Illustration of DSC thermograms of native folding and aggregation. Pane A: The continuous line represents a Cp curve of rmIII-9 observed and the dotted line indicates a best fit of the peak to a single two-state thermal transition (see Figure 5B of Litvinovich et al. [23] for more details). Pane B: First and second heating of ARQ are shown with continuous and dotted lines, respectively (see Figures 3C and 4D of Rezaei et al. for ARQ [25] for more details). Pane C: DSC thermograms of FNR. First and second heat scans are shown with continuous and dotted lines, respectively. Pane D: Top views of test tubes containing FNR sample solutions before (upper) and after (lower) DSC measurements on the right. Light scattering intensities of 25 mM sodium phosphate buffer (pH 7.4) alone, and FNR samples before and after DSC measurements on the left.
Similar aggregation-related thermal responses in DSC thermograms [24] were interpreted by Conejero-Lara et al. in the year 2002. In order to demonstrate the occurrence of aggregation and irreversible thermal unfolding induced by aggregation, deformed Cp curves for the thermal unfolding of streptokinase enzyme at high protein concentrations or mildly acidic pH were employed.
Conejero-Lara et al. argued that kinetic controls (that is, a scanning rate dependence) caused by the slow exchange between aggregates and thermally-unfolded soluble streptokinase hindered the canonical thermodynamic DSC analysis.
Despite this fact, when the DSC profiles were fitted to model equations, it was possible to obtain a number of thermodynamic parameters of streptokinase aggregation, for example ΔΔG and ΔH from the unfolded states to the aggregation states.
In the same year, DSC studies were carried out by Rezaei and colleagues on the association between disease resistance/susceptibility and the stabilities of many variants of the prion protein (PrP). This protein is closely associated with neurodegenerative pathologies such as transmissible spongiform encephalopathies, and prion diseases[25].
ARQ is a disease-susceptible variant. Heat-induced unfolding of this variant was irreversible owing to the formation of fibrillar aggregates following repeated DSC scans, that is, repeating the cycle of heating and successive cooling down, as depicted in Figure 2B.
Exothermic peaks with no endothermic peak were shown by the second DSC up-scan. While the origins of heat-generating processes were not fully explained by the authors, the generation of fibrillar aggregates after the first DSC scan can be attributed to these exothermic transition peaks.
Therefore, DSC thermograms with exothermic responses can be applied to detect the presence of aggregates, aggregation, or a combination of these both.
Ferredoxin NADP+ reductase (FNR) is a large (~35.5 kDa) multi-domain enzyme [11]. DSC measurements of this FNR were carried out to study the origins of exothermic peaks. The initial DSC scan of FNR was performed which revealed an exothermic response after an endothermic DSC peak for the thermal unfolding process, but the second DSC scan of FNR did not show a characteristic peak in the Cp curve, as illustrated in Figure 2C.
Following DSC measurements, the sample solution was found to be cloudy and showed a high intensity of light scattering because of aggregate formation, as shown in Figure 2D. This indicates a relationship between the Cp curve exothermic pattern following thermal denaturation of proteins and aggregation and precipitation of proteins.
Exothermic response of amyloid fibrillation and amyloid fibrils on heating
In 2003 and 2005, Dzwolak and Winter et al. showed clearer DSC profiles for the formation of amyloids [26,27].
The authors explicitly showed that the formation of amyloids by insulin, which, in turn, induces injection-associated amyloidosis, was further accompanied by an exothermic transition following the endothermic thermal unfolding mechanism of native folds, irrespective of changes in the experimental conditions (Figure 3) [26,27]. The Cp curves of the formation of insulin amyloid relied on:
- the scan rate (i.e., kinetic controls) (Figure 3A)
- temperature (Figure 3B)
- protein (Figure 3C)
- alcohol concentrations (Figure 3D)
The obvious ΔH for insulin amyloid formation at differing temperatures between 60 and 75 °C and ethanol concentrations from 0 to 30% were -16 – -57 kJ mol-1 as shown in Figure 3B, and -70 – -140 kJ mol-1 as indicated in Figure 3D.
![DSC scans of insulin aggregation at pH 1.9 under various experimental conditions. (A) DSC thermograms of insulin at distinct scan rates (10 (solid line), 20 (dotted line), and 10 °C h-1 (dashed dotted line)) [26]. (B) Heat obtained at 80 (curve 1), 75 (curve 2), 70 (curve 3), 65 (curve 4), 60 (curve 5), and 55 °C (curve 6) [26]. (C) DSC profiles obtained at 1 (solid line), 2 (dotted line), and 3% (dash dot line) with a scan rate of 20 °C h-1[26]. (D) DSC curves of 0.5 wt % insulin obtained at various ethanol concentrations, which are indicated in the figure [27].](https://www.news-medical.net/image-handler/picture/2017/7/3_Malvern.jpg)
Figure 3. DSC scans of insulin aggregation at pH 1.9 under various experimental conditions. (A) DSC thermograms of insulin at distinct scan rates (10 (solid line), 20 (dotted line), and 10 °C h-1 (dashed dotted line)) [26]. (B) Heat obtained at 80 (curve 1), 75 (curve 2), 70 (curve 3), 65 (curve 4), 60 (curve 5), and 55 °C (curve 6) [26]. (C) DSC profiles obtained at 1 (solid line), 2 (dotted line), and 3% (dash dot line) with a scan rate of 20 °C h-1[26]. (D) DSC curves of 0.5 wt % insulin obtained at various ethanol concentrations, which are indicated in the figure [27].
Using a number of amyloidogenic proteins and peptides, Sasahara and colleagues performed a set of DSC-based aggregation studies between 2005 and 2009 (Figure 4) [28-32]. The authors assessed two types of apparent exothermic heat:
- the seed-reliant amyloid formation of β2-microglobulin (β2-m) which causes dialysis-related amyloidosis, as shown in Figure 4A [29, 32]
- amyloid fibrils of β2-m and amyloid β (Aβ) peptides/fragments responsible for Alzheimer’s disease, as depicted in Figure 4B [28]
![DSC scans for monitoring heat responses of amyloid fibrillation, amyloid fibrils, and conversion of aggregation states. Pane A: DSC thermograms of β2-m amyloid formation at various scan rates (20 °C h-1 (curve 1), 30 °C h-1 (curve 2), 50 °C h-1 (curve 3), 70 °C h-1 (curve 4), and 90 °C h-1 (curve 6)) with 0.2 mg mL-1 acidic-denatured β2-m and 0.5 mg mL-1 β2-m seed fibrils. The upper flat black curve indicates second heating after first heating to 120 °C [29]. Pane B: Schematic illustration of DSC traces of the heating of β2-m or Aβ(1-40) amyloid fibrils at varying protein concentrations. Increases in the concentration of β2-m or Aβ(1-40) are shown with numbering from 1 to 6 (see Figures 1A (β2-m) and 6A (Aβ(1-40)) of Sasahara et al. [28] for more details). Pane C: DSC thermograms of β2-m protofibrils formed at 0.5 M NaCl at a scan rate of 60 °C h-1[31]. Pane D: DSC thermograms of HEWL amorphous aggregates were depicted (see Figure 5A of Sasahara et al. [32] for more details). Curve 1 indicates the Cp curve of native HEWL. The number in curves 2 and 3 indicates the order of DSC heat scans. (E) Heat flow of the transition from spherical to tubular aggregates of diphenylalanine at the desired temperature [37].](https://www.news-medical.net/image-handler/picture/2017/7/4_Malvern.jpg)
Figure 4. DSC scans for monitoring heat responses of amyloid fibrillation, amyloid fibrils, and conversion of aggregation states. Pane A: DSC thermograms of β2-m amyloid formation at various scan rates (20 °C h-1 (curve 1), 30 °C h-1 (curve 2), 50 °C h-1 (curve 3), 70 °C h-1 (curve 4), and 90 °C h-1 (curve 6)) with 0.2 mg mL-1 acidic-denatured β2-m and 0.5 mg mL-1 β2-m seed fibrils. The upper flat black curve indicates second heating after first heating to 120 °C [29]. Pane B: Schematic illustration of DSC traces of the heating of β2-m or Aβ(1-40) amyloid fibrils at varying protein concentrations. Increases in the concentration of β2-m or Aβ(1-40) are shown with numbering from 1 to 6 (see Figures 1A (β2-m) and 6A (Aβ(1-40)) of Sasahara et al. [28] for more details). Pane C: DSC thermograms of β2-m protofibrils formed at 0.5 M NaCl at a scan rate of 60 °C h-1[31]. Pane D: DSC thermograms of HEWL amorphous aggregates were depicted (see Figure 5A of Sasahara et al. [32] for more details). Curve 1 indicates the Cp curve of native HEWL. The number in curves 2 and 3 indicates the order of DSC heat scans. (E) Heat flow of the transition from spherical to tubular aggregates of diphenylalanine at the desired temperature [37].
The DSC thermograms of amyloid formation are highly complex and were inferred to reflect various processes, as shown in Figure 4A) [28-32]. The growth of amyloid fibrils (1st step) causes the first sigmoidal exothermic effect and this was followed by a slow decrease in Cp curves owing to the relationship among amyloid fibrils (2nd step) — a process under kinetic controls depending on scan rate-dependent increases in exothermic effects, as indicated in Figure 4A and Figure B. A significant increase in Cp curves (i.e., endothermic reaction) for amyloid fibril melting was successively detected in the 3rd step after the minimum Cp value.
This exceptional interpretation was sustained by kinetic controls and analogous negative DSC peaks in the DSC thermograms of the above-mentioned preformed amyloid fibrils of the Aβ peptide and β2- m (Figure 4B) and the heat denaturation of amyloid fibrils detected using far-UV CD spectroscopy [28]. In the DSC thermograms of preformed amyloid fibrils, the 2nd and 3rd steps were found to be quite analogous to those of seed-reliant amyloid formation. Further, the 2nd step was scan-rate dependent and reversible, which assigned the 1st step to amyloid fibril formation. Also, the onset temperature established for melting of preformed amyloid fibrils, which were identified by far-UV CD spectroscopy, was found to be consistent with the temperature for the least Cp value, which happens to be the initial point for the 3rd step.
One can infer the exothermic nature of amyloid formation and endothermic melting in the 1st and 3rd steps, respectively. It is in the 2nd step that the origin of the kinetic thermal responses for the exothermic effects is seen and this may include transient and specific interactions between dehydration [32], amyloid fibrils, and the trapping of water, which reduces water motion [33]. Consecutive scans revealed exothermic responses in DSC profiles as a result of the formation of α-crystalline amyloid fibrils [34]. Also, Guzzi et al. reported the formation of exothermic amyloids by β-lactoglobulin in the presence and absence of Zn2+ or Cu2+ [35,36].
Exothermic heat for the conversion of aggregation states
Different types of aggregates exhibit exothermic transitions [31,32,37]. Protofibrils of acid-denatured β2-m were prepared through agitation at a high NaCl (0.5 M) concentration. These protofibrils were transformed to mature amyloid fibrils as highlighted by the reduction in the Cp curve (that is an exothermic reaction) as shown in Figure 4C [31,32]. Next, hen egg white lysozyme (HEWL) at pH 2-6 and amorphous aggregates of native-like structures of β2-m at neutral pH were converted to mature amyloid fibrils, as indicated by exothermic responses in DSC scans illustrated in Figure 4D [30,32].
Through incubation in the DSC cell at a set temperature, the spherical assemblies of diphenylalanine were changed into highly-ordered tubular aggregates, as shown in Figure 4E [37]. These diphenylalanine can be considered as amorphous aggregates. In addition, this phase transition produced distinct negative exothermic peaks with incubation times set at a number of temperatures (that is isoscan mode in VP-DSC (MicroCalTM from Malvern Panalytical, UK).
Melting of fibrillar aggregates with endothermic heat
Disaggregation or unfolding process may involve the uptake of heat when compared to the exothermic reactions, which are employed to arrange the protein structures by aggregation induced by folding or misfolding processes. Positive endothermic DSC peaks were revealed by the disassembly of preformed amyloid fibrils[38-40]. The melting of amyloid fibrils that were formed from the N-terminal prion domain of Ure2p [38] and N47A[39,40] — a variant of the α-spectrin SH3 domain — resulted in the absorption of heat, as shown in Figure 5A-C. Thermodynamic analyses were then performed based on clear transition peaks. Also, ΔH and TM for the heat denaturation of N47A and Ure2p amyloids were ~75 °C – ~90 °C and ~100 kJ mol-1 – ~130 kJ mol-1 and ~75 °C and ~370 kJ mol-1, respectively. Protofibrils that formed from acid-denatured β2-m at NaCl concatenation of 0.5 M were thermally depolymerized, as illustrated by a single endothermic peak in Figure 5D [29,31].
![DSC scans showing the melting of protein aggregates. Pane A: DSC thermograms of soluble and aggregated Ure2p in 20 mM sodium phosphate buffer (pH 7.6) containing 100 mM NaCl. Samples were heated at 120 °C h-1[38]. Pane B: DSC curves of the N47A variant of the SH3 domain at 8.3 mg mL-1 in 100 mM glycine buffer (pH 3.2) containing 100 mM NaCl. Pre-incubation times are shown. Transition peaks at higher temperatures resulted from the melting of N47A amyloid fibrils [39]. Pane C: Cp curves of the amyloid fibrils of N47A at 5.5 mg mL-1 prepared in 100 mM glycine buffer for 1 month with distinct scan rates [40]. Pane D: Melting of β2-m protofibrils formed at 0.5 M NaCl scanned at 60 °C h-1[29]. β2-m concentrations were 0.2 mg mL-1 (curve 1), 0.3 mg mL-1 (curve 2), and 0.5 mg mL-1 (curve 3). It should be noted that Figure 5A and B were reproduced based on original papers [38,39].](https://www.news-medical.net/image-handler/picture/2017/7/5_Malvern.jpg)
Figure 5. DSC scans showing the melting of protein aggregates. Pane A: DSC thermograms of soluble and aggregated Ure2p in 20 mM sodium phosphate buffer (pH 7.6) containing 100 mM NaCl. Samples were heated at 120 °C h-1[38]. Pane B: DSC curves of the N47A variant of the SH3 domain at 8.3 mg mL-1 in 100 mM glycine buffer (pH 3.2) containing 100 mM NaCl. Pre-incubation times are shown. Transition peaks at higher temperatures resulted from the melting of N47A amyloid fibrils [39]. Pane C: Cp curves of the amyloid fibrils of N47A at 5.5 mg mL-1 prepared in 100 mM glycine buffer for 1 month with distinct scan rates [40]. Pane D: Melting of β2-m protofibrils formed at 0.5 M NaCl scanned at 60 °C h-1[29]. β2-m concentrations were 0.2 mg mL-1 (curve 1), 0.3 mg mL-1 (curve 2), and 0.5 mg mL-1 (curve 3). It should be noted that Figure 5A and B were reproduced based on original papers [38,39].
It is important to consider limitations related to the application of DSC for studying protein aggregation. When a DSC technique is used, precise and accurate estimation of the thermodynamic parameters of protein aggregation becomes very difficult due to constant temperature fluctuations during aggregation.
Also, the pathway of aggregation and stability of aggregates are temperature-dependant. Unreliable DSC thermograms can occur owing to low reproducibility and large noise arising from the precipitation of bulk protein aggregates as well as the sticky aggregates that tend to stick to a DSC cell surface. Therefore, more careful study and more credible proof are required to understand the kinetic thermal responses.
More systematic case studies using DSC can be used to describe the actual mechanism involved in the unique thermal response of protein aggregation. Yet, the above-described findings deliver a rapid qualitative judgement regarding the respective formation and removal of amyloid fibrils by merely observing the positive and negative DSC peaks beyond quantitative assessments.
Thermodynamic study of protein aggregation using ITC
Instead of DSC, ITC can be used to study the aggregation of proteins using its controlled titration and stirring function at a steady temperature. Compared to DSC, ITC can be predicted to generate more reproducible and reliable data on protein aggregation because aggregate solutions are dispersed by continuous stirring and precipitation is prevented.
As a result, reaction heat is efficiently transferred to thermal sensors. It was shown that in addition to being a powerful approach for studying protein aggregation, ITC also enables the thermodynamic characterization of protein aggregation in a precise and accurate manner.
Examination of seed-dependent amyloid formation using ITC
Only limited amount of data is available on ITC-based investigations of protein aggregation [41-43]. In 2004 Kardos et al. conducted the first trial and successfully identified the exothermic heat generated during the elongation of β2-m amyloid fibrils through VP-ITC (MicroCalTM from Malvern Panalytical, UK) and then thermodynamically defined the aggregation of protein, as shown in Figure 6A and B [41].
In the ITC syringe, preformed β2-m amyloid fibrils (that is seed fibrils) were titrated into acid-denatured β2-m monomers present within the ITC cell for single monitoring of the reaction of fibril growth, as illustrated in Figure 6A.
In the ITC syringe, acid-denatured β2-m monomers were also titrated into β2-m seed fibrils present in the ITC cell for continuous observations of fibril growth reactions in one ITC experiment. This is done because separate injections promote fibril elongation.
124 kJ mol-1 was the ΔH value achieved at 37 °C and this differed in a temperature-dependent way. For β2-m fibril growth, the ΔCp value was inferred from ΔH’s temperature dependence, as shown in Figure 6B.
It was estimated that the ΔS and ΔG of amyloid fibrils are 80 kJ mol-1 and -43.9 kJ mol-1 at 37 °C, respectively depending on remaining monomers within the two-state equilibrium between fibrils and monomers; the relationship, ΔG = ΔH - TΔS = -RTlnK, where K and R represent the equilibrium constant and gas constant, respectively. The driving forces for formation of amyloids at 37 °C were positive ΔS and negative ΔH.
![Observation of heat and temperature-dependent ΔH for the growth of amyloid fibrils using ITC. Pane A: ITC thermograms recorded by the injection of seeds at a final concentration of 15 μg mL-1 in the ITC syringe to a solution of β2m monomers at 0.1 mg mL-1 in the ITC cell at 37 °C (line 1). Overlapping thin lines indicate a series of reference measurements as buffer to buffer (line 2), buffer to monomer (line 3), and seed to buffer solution (line 4) [41]. Pane B: Each ΔH (black sphere) of the β2m fibril extension was plotted as a function of temperature. The black line indicates the best fit curve with a straight line [41]. Pane C: A schematic representation of ITC thermograms of the growth of glucagon amyloid fibrils in sulfate buffer containing 10 mM HCl and 1 mM Na2SO4, obtained by increasing temperatures from 17 °C to 45 °C (see Figure 1A of Jeppesen et al. [42] for more details). Pane D: Temperature dependence of ΔH measured in the three distinct buffers (5 mM HCl solution containing 150 mM NaCl (solid line), 50 mM glycine buffer (pH 2.5) containing 1 mM sulfate (broken line), and 50 mM glycine buffer (pH 2.5) (dotted line)) are schematically shown (see Figure 1B of Jeppesen et al. [42] for more details).](https://www.news-medical.net/image-handler/picture/2017/7/6_Malvern.jpg)
Figure 6. Observation of heat and temperature-dependent ΔH for the growth of amyloid fibrils using ITC. Pane A: ITC thermograms recorded by the injection of seeds at a final concentration of 15 μg mL-1 in the ITC syringe to a solution of β2m monomers at 0.1 mg mL-1 in the ITC cell at 37 °C (line 1). Overlapping thin lines indicate a series of reference measurements as buffer to buffer (line 2), buffer to monomer (line 3), and seed to buffer solution (line 4) [41]. Pane B: Each ΔH (black sphere) of the β2m fibril extension was plotted as a function of temperature. The black line indicates the best fit curve with a straight line [41]. Pane C: A schematic representation of ITC thermograms of the growth of glucagon amyloid fibrils in sulfate buffer containing 10 mM HCl and 1 mM Na2SO4, obtained by increasing temperatures from 17 °C to 45 °C (see Figure 1A of Jeppesen et al. [42] for more details). Pane D: Temperature dependence of ΔH measured in the three distinct buffers (5 mM HCl solution containing 150 mM NaCl (solid line), 50 mM glycine buffer (pH 2.5) containing 1 mM sulfate (broken line), and 50 mM glycine buffer (pH 2.5) (dotted line)) are schematically shown (see Figure 1B of Jeppesen et al. [42] for more details).
Using the same ITC method, Jeppesen et al. noticed the heat of the elongation reaction of fibrils that were formed by a peptide hormone called glucagon (Figure 6C) [42].
The authors acquired ΔCp and ΔH values for growth of glucagon fibrils and showed the polymorphic characteristic of glucagon fibrillation in terms of the varying thermodynamic responses (i.e. the magnitude and sign) of ΔCp and ΔH based on solvent conditions (that is types of salts), which, in turn, revealed yet another practical application for ITC in addition to thermodynamic analyses as shown in Figure 6D [42].
Direct observations of the heat of spontaneous protein aggregation using ITC
Recently, Ikenoue and Lee et al. exclusively applied VP-ITC (MicroCalTM from Malvern Panalytical, UK) for monitoring the heat of spontaneous formation of amyloids as well as amorphous aggregation of β2-m in real time (Figure 7) [44].
The authors reported that precise and accurate thermodynamic characterization of protein aggregation based on calorimetry can be achieved, and they further characterized the clear conformational states of of β2-m i.e. natively folded states and aggregated states of β2-m induced by misfolding.
![Monitoring the heat of the spontaneous amyloid formation of β2m using ITC and other approaches [44]. Pane A: The ITC trace of β2-m (1.1 mg mL-1) at pH 2.5 and 37 °C (upper panel). Conformational states of β2-m during an incubation in the ITC cell examined using AFM images and thioflavin T (ThT) fluorescence intensities (middle panel) and far-UV CD spectra (lower panel) at the four time points (0 hours, ~3.5 hours, ~5.5 hours, and ~12 hours). Illustrations of the molecular species of β2m based on AFM, ThT fluorescence, and CD are shown above AFM images: monomers (blue curves), prefibrillar aggregates (magenta curves), and amyloid fibrils (red rectangles). The CD spectra at each time point are shown by red solid curves. As a comparison, the spectra of monomers and mature fibrils (red dotted curves) are shown with black and red dotted curves, respectively. Pane B: Temperature-dependent ΔH for amorphous aggregation (blue circles), spontaneous (red triangles) and seed-dependent fibrillation (black squares), and folding (black circles) (left) are shown. The difference in ΔH at 60 °C among the distinct conformational states is shown with animation (right).](https://www.news-medical.net/image-handler/picture/2017/7/7_Malvern.jpg)
Figure 7. Monitoring the heat of the spontaneous amyloid formation of β2m using ITC and other approaches [44]. Pane A: The ITC trace of β2-m (1.1 mg mL-1) at pH 2.5 and 37 °C (upper panel). Conformational states of β2-m during an incubation in the ITC cell examined using AFM images and thioflavin T (ThT) fluorescence intensities (middle panel) and far-UV CD spectra (lower panel) at the four time points (0 hours, ~3.5 hours, ~5.5 hours, and ~12 hours). Illustrations of the molecular species of β2m based on AFM, ThT fluorescence, and CD are shown above AFM images: monomers (blue curves), prefibrillar aggregates (magenta curves), and amyloid fibrils (red rectangles). The CD spectra at each time point are shown by red solid curves. As a comparison, the spectra of monomers and mature fibrils (red dotted curves) are shown with black and red dotted curves, respectively. Pane B: Temperature-dependent ΔH for amorphous aggregation (blue circles), spontaneous (red triangles) and seed-dependent fibrillation (black squares), and folding (black circles) (left) are shown. The difference in ΔH at 60 °C among the distinct conformational states is shown with animation (right).
In the ITC syringe, NaCl was slowly titrated to acid-denatured β2-m monomers present in the ITC cell so as to achieve a final concentration of 100 mM. As the NaCl concentration in the ITC cell was gradually increased, the solubility of β2-m monomers decreased and the metastability of supersaturation was changed, which, in turn, created a lag time.
Huge amounts of exothermic heat were accompanied by a lag time following salt titration, as shown in Figure 7A, upper panel. Due to this lag time, it was possible to separate exothermic fibrillation heat from the huge endothermic peaks of the salt injection’s dilution heat.
This huge exothermic heat was the result of the formation of amyloid fibrils, as shown by the data obtained from other approaches such as a thioflavin T assay, atomic force microcopy, and far-UV CD spectroscopy indicated in Figure 7A, middle and lower panels.
Using continuous stirring as an efficient agitation, β2-m monomers were released from the kinetically-bound supersaturated states of β2-m through the metastability of supersaturation, thus making effective nuclei for formation of amyloids.
At 37 °C, ΔH was about -80 kJ mol-1. As temperature was reduced, the magnitude of ΔH also decreased as noted for β2-m fibril growth. In addition, ΔCp was estimated from the ΔH plot slope versus temperature, as shown in Figure 7B, left.
The thermodynamics of amorphous aggregation of β2-m at high NaCl concentration was assessed by carrying out reverse titration. The ITC syringe continued monomeric β2-m solution that was administrated into the ITC cell comprising of 1.0 M NaCl.
Owing to the low metastability of supersaturation, reaction heat was noted without a clear lag time and then amorphous aggregation was validated using a number of techniques after reactions. At 37 °C, ΔH was about -40 kJ mol-1 and ΔCp from ΔH’s linear temperature dependence was also achieved, as illustrated in Figure 7B, left. In the same way, ΔS and ΔG were achieved as seed-dependent amyloid fibrillation.
ITC provided thermodynamic parameters based on which the small conformational states of β2m were characterized in clear conformations, that is, amyloid fibrillar states, natively folded states, and amorphous aggregated states, as shown in Figure 7B, left, which were not compatible with other methods.
The DCp of the conversions of unfolded β2-m state into three conformational states were all observed to be similar (~ -3.5 kJ mol-1 K-1 - ~ -5.5 kJ mol-1 K-1), indicating an analogous buried accessible surface region between folding and aggregation of β2-m. Therefore, ΔH for folding and aggregation of β2-m was attributed to variations in internal interactions.
At 60 °C, the order of the magnitude of ΔH was: native folds (~ -300 kJ mol-1), followed by amyloid fibrils (~ -200 kJ mol-1), and then by amorphous aggregates (~ -120 kJ mol-1) as shown in Figure 7B, right. This indicates that the internal packing of natively-folded β2-m achieved by improved side-chain interactions was found to be higher than the misfolded aggregates of β2-m that were predominantly produced by main-chain interactions.
Amorphous aggregates lack specific internal interactions. Amyloid fibrils that are highly ordered and contain internal hydrogen bond networks are more tightly packed states when compared to these amorphous aggregates.
Conclusion
Some of the key issues faced in many research fields are misfolding and aggregation of proteins. Robust calorimetric techniques have been used to expose the thermodynamic properties and the underlying mechanisms of misfolding and aggregation of proteins.
Additional data regarding the thermodynamic behavior of abnormal protein aggregation may be acquired by increasing the merits of ITC and DSC. While complete elucidation of the thermodynamic behavior of protein misfolding and aggregation is yet to be achieved, additional case studies will help establish the thermodynamics of protein aggregates related to diseases along with the thermodynamic behavior of protein folding.
Aside from the comprehensive quantitative and qualitative examinations on the thermodynamic behavior of protein aggregation, heat itself serves as a fast and simple indicator for evaluating the type and formation of aggregates by observing the magnitude and sign of the reaction heat on thermograms.
Also, these reaction heat properties, i.e., sign, magnitude, pattern, and shape of the thermogram will be relevant to kinetics and also to the development of inhibitors of protein aggregation.
Acknowledgments
We thank Professor Yuji Goto (Osaka University, Japan) and Associate Professor József Kardos (Eö tvö s Lorań d University, Hungary) for their comments on calorimetric studies. We thank Dr. Natalia Markova (Malvern Panalytical, UK) for critical reading of this manuscript. We also thank Ms. Masako Hirose and Ms. Takuma Waki (Malvern Panalytical, Japan) for their kind support of our calorimetric studies.
Produced from materials originally authored by Tatsuya Ikenoue*, Misaki Kinoshita**, Yuxi Lin**, and Young-Ho Lee.** from:
*Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK.
**Institute for Protein Research, Osaka University, Yamadaoka 3-2, Suita, Osaka 565-0871, Japan
References
- Fersht, A. R. and Daggett, V. (2002) Protein folding and unfolding at atomic resolution. Cell 108, 573-582
- Daggett, V. and Fersht, A. (2003) The present view of the mechanism of protein folding. Nat Rev Mol Cell Biol 4, 497-502
- Baldwin, R. L. (2007) Energetics of protein folding. J Mol Biol 371, 283-301
- Nickson, A. A. and Clarke, J. (2010) What lessons can be learned from studying the folding of homologous proteins? Methods 52, 38-50
- Jelesarov, I. and Bosshard, H. R. (1999) Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J Mol Recognit 12, 3-18
- Leavitt, S. and Freire, E. (2001) Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr Opin Struct Biol 11, 560-566
- Markova, N. and Hallen, D. (2004) The development of a continuous isothermal titration calorimetric method for equilibrium studies. Anal Biochem 331, 77-88
- Velazquez Campoy, A. and Freire, E. (2005) ITC in the post-genomic era...? Priceless. Biophys Chem 115, 115-124
- Privalov, P. L. (2009) Microcalorimetry of proteins and their complexes. Methods Mol Biol 490, 1-39
- Ladbury, J. E. (2010) Calorimetry as a tool for understanding biomolecular interactions and an aid to drug design. Biochem Soc Trans 38, 888-893
- Lee, Y. H., Ikegami, T., Standley, D. M., Sakurai, K., Hase, T. and Goto, Y. (2011) Binding energetics of ferredoxin-NADP+ reductase with ferredoxin and its relation to function. Chembiochem 12, 2062-2070
- Powers, E. T., Morimoto, R. I., Dillin, A., Kelly, J. W. and Balch, W. E. (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78, 959-991
- Lee, Y. H. and Goto, Y. (2012) Kinetic intermediates of amyloid fibrillation studied by hydrogen exchange methods with nuclear magnetic resonance. Biochim Biophys Acta 1824, 1307-1323
- Knowles, T. P., Vendruscolo, M. and Dobson, C. M. (2014) The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol 15, 384-396
- Yoshimura, Y., Lin, Y., Yagi, H., Lee, Y. H., Kitayama, H., Sakurai, K., So, M., Ogi, H., Naiki, H. and Goto, Y. (2012) Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation. Proc Natl Acad Sci U S A 109, 14446-14451
- Lin, Y., Lee, Y. H., Yoshimura, Y., Yagi, H. and Goto, Y. (2014) Solubility and supersaturation-dependent protein misfolding revealed by ultrasonication.Langmuir 30, 1845-1854
- Muta, H., Lee, Y. H., Kardos, J., Lin, Y., Yagi, H. and Goto, Y. (2014) Supersaturation-limited amyloid fibrillation of insulin revealed by ultrasonication. J Biol Chem 289, 18228-18238
- Lin, Y., Kardos, J., Imai, M., Ikenoue, T., Kinoshita, M., Sugiki, T., Ishimori, K., Goto, Y. and Lee, Y. H. (2016) Amorphous Aggregation of Cytochrome c with Inherently Low Amyloidogenicity Is Characterized by the Metastability of Supersaturation and the Phase Diagram. Langmuir 32, 2010-2022
- Privalov, P. L. and Dragan, A. I. (2007) Microcalorimetry of biological macromolecules. Biophys Chem 126, 16-24
- Privalov, P. L. (2007) Reflections on the origins of microcalorimetry of biopolymers. Biophys Chem 126, 13-15
- Iida, T., Nishimura, S., Mochizuki, M., Uchiyama, S., Ohkubo, T., Urade, Y., Tanaka, A. and Inui, T. (2008) Thermal unfolding mechanism of lipocalin-type prostaglandin D synthase. FEBS J 275, 233-241
- Schon, A. and Freire, E. (2016) Three easy pieces. Biochim Biophys Acta 1860, 975-980
- Litvinovich, S. V., Brew, S. A., Aota, S., Akiyama, S. K., Haudenschild, C. and Ingham, K. C. (1998) Formation of amyloid-like fibrils by self-association of a partially unfolded fibronectin type III module. J Mol Biol 280, 245-258
- Azuaga, A. I., Dobson, C. M., Mateo, P. L. and Conejero-Lara, F. (2002) Unfolding and aggregation during the thermal denaturation of streptokinase. Eur J Biochem 269, 4121-4133
- Rezaei, H., Choiset, Y., Eghiaian, F., Treguer, E., Mentre, P., Debey, P., Grosclaude, J. and Haertle, T. (2002) Amyloidogenic unfolding intermediates differentiate sheep prion protein variants. J Mol Biol 322, 799-814
- Dzwolak, W., Ravindra, R., Lendermann, J. and Winter, R. (2003) Aggregation of bovine insulin probed by DSC/PPC calorimetry and FTIR spectroscopy. Biochemistry 42, 11347-11355
- Dzwolak, W., Grudzielanek, S., Smirnovas, V., Ravindra, R., Nicolini, C., Jansen, R., Loksztejn, A., Porowski, S. and Winter, R. (2005) Ethanol-perturbed amyloidogenic self-assembly of insulin: looking for origins of amyloid strains. Biochemistry 44, 8948-8958
- Sasahara, K., Naiki, H. and Goto, Y. (2005) Kinetically controlled thermal response of beta2-microglobulin amyloid fibrils. J Mol Biol 352, 700-711
- Sasahara, K., Naiki, H. and Goto, Y. (2006) Exothermic effects observed upon heating of beta2-microglobulin monomers in the presence of amyloid seeds. Biochemistry 45, 8760-8769
- Sasahara, K., Yagi, H., Naiki, H. and Goto, Y. (2007) Heat-induced conversion of beta(2)-microglobulin and hen egg-white lysozyme into amyloid fibrils. J Mol Biol 372, 981-991
- Sasahara, K., Yagi, H., Naiki, H. and Goto, Y. (2007) Heat-triggered conversion of protofibrils into mature amyloid fibrils of beta2-microglobulin. Biochemistry 46, 3286-3293
- Sasahara, K., Yagi, H., Naiki, H. and Goto, Y. (2009) Thermal response with exothermic effects of beta2-microglobulin amyloid fibrils and fibrillation. J Mol Biol 389, 584-594
- Bergqvist, S., Williams, M. A., O'Brien, R. and Ladbury, J. E. (2004) Heat capacity effects of water molecules and ions at a protein-DNA interface. J Mol Biol 336, 829-842
- Attanasio, F., Cataldo, S., Fisichella, S., Nicoletti, S., Nicoletti, V. G., Pignataro, B., Savarino, A. and Rizzarelli, E. (2009) Protective effects of L- and Dcarnosine on alpha-crystallin amyloid fibril formation: implications for cataract disease. Biochemistry 48, 6522-6531
- Stirpe, A., Rizzuti, B., Pantusa, M., Bartucci, R., Sportelli, L. and Guzzi, R.(2008) Thermally induced denaturation and aggregation of BLG-A: effect of the Cu(2+) and Zn (2+) metal ions. Eur Biophys J 37, 1351-1360
- Guzzi, R., Rizzuti, B., Labate, C., Zappone, B. and De Santo, M. P. (2015) Ferric Ions Inhibit the Amyloid Fibrillation of beta-Lactoglobulin at High Temperature. Biomacromolecules 16, 1794-1801
- Levin, A., Mason, T. O., Adler-Abramovich, L., Buell, A. K., Meisl, G., Galvagnion, C., Bram, Y., Stratford, S. A., Dobson, C. M., Knowles, T. P., et al. (2014) Ostwald's rule of stages governs structural transitions and morphology of dipeptide supramolecular polymers. Nat Commun 5, 5219
- Baxa, U., Ross, P. D., Wickner, R. B. and Steven, A. C. (2004) The N-terminal prion domain of Ure2p converts from an unfolded to a thermally resistant conformation upon filament formation. J Mol Biol 339, 259-264
- Morel, B., Casares, S. and Conejero-Lara, F. (2006) A single mutation induces amyloid aggregation in the alpha-spectrin SH3 domain: analysis of the early stages of fibril formation. J Mol Biol 356, 453-468
- Morel, B., Varela, L. and Conejero-Lara, F. (2010) The thermodynamic stability of amyloid fibrils studied by differential scanning calorimetry. J Phys Chem B 114, 4010-4019
- Kardos, J., Yamamoto, K., Hasegawa, K., Naiki, H. and Goto, Y. (2004) Direct measurement of the thermodynamic parameters of amyloid formation by isothermal titration calorimetry. J Biol Chem 279, 55308-55314
- Jeppesen, M. D., Hein, K., Nissen, P., Westh, P. and Otzen, D. E. (2010) A thermodynamic analysis of fibrillar polymorphism. Biophys Chem 149, 40-46
- Ikenoue, T., Lee, Y. H., Kardos, J., Saiki, M., Yagi, H., Kawata, Y. and Goto, Y. (2014) Cold denaturation of alpha-synuclein amyloid fibrils. Angew Chem Int Ed Engl 53, 7799-7804
- Ikenoue, T., Lee, Y. H., Kardos, J., Yagi, H., Ikegami, T., Naiki, H. and Goto, Y. (2014) Heat of supersaturation-limited amyloid burst directly monitored by isothermal titration calorimetry. Proc Natl Acad Sci U S A 111, 6654-6659
About Malvern Panalytical

Malvern Panalytical provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems.
These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries.
Used at all stages of research, development and manufacturing, Malvern Panalytical’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.