A recent report suggested that a typical pharmaceutical manufacturing factory will spend approximately $40M on quality control every year.1 Meanwhile, another report highlighted that 79% of the 483 Warning Letters issued by the FDA in 2016 cited 21 CFR part 11 data integrity issues.2
An FDA 483 Warning Letter will often prompt retraining within an organisation, but this may be only one part of the solution. It could be that the worker(s) did not follow the SOP correctly, or it could be the case that manual SOPs are not robust enough to prevent the problem reoccurring in the future.
Processes which automate the quality control SOP have the potential to reduce the impact of human error on data integrity, while reducing the time needed to carry out the SOP – leading to lower costs and less technician time required.
This article examines four common quality control procedures, exploring the way in which automation can improve data integrity via reduced opportunities for human error, whilst also reducing operating costs and saving time.
These four quality control procedures form a common thread in the biopharmaceutical manufacturing industry:
- Water quality testing
- Cleanroom routine environmental monitoring
- Biological cell counting and viability testing
- Final injectable drug particulate testing
Introduction
Despite FDA encouragement via their 2004 PAT initiative,3 a number of the pharmaceutical industry’s routine quality control (QC) test procedures are undertaken manually, making them extremely time consuming.
A study conducted by the Industrial BioDevelopment Laboratory revealed >20% variability in test results when 16 experienced QC technicians performed an identical manual SOP.4 While the manual SOP may be enhanced and the technicians in question retrained, this level of variability in results indicates that it is the manual process itself which is not robust enough to guarantee the necessary levels of quality control.
The technical specifications of similar equipment may seem equivalent, but quality control QC teams should consider the instrument’s level of automation when looking at ways to make QC testing more repeatable and robust.
For example, a typical cause of errors in QC testing stems from sample preparation. An instrument which is able to automate the sample preparation process of a QC test could provide considerable improvements in reproducibility and robustness.4
After the implementation of the FDA PAT initiative, users might contemplate a shift from sole reliance on QC testing in a laboratory, to the use of on-line QC instrumentation.
For example, a large number of companies make use of validated on-line water quality testing instrumentation as part of their Water for Injection (WFI) and Purified Water (PW) loops. However, these companies still rely on laborious laboratory water testing when testing product releases.
If it is possible to validate on-line instruments to meet the requirements of the pharmacopoeias, reducing the amount of laboratory testing is worthwhile, instead relying on data from on-line instruments.
As these on-line instruments are automated, this in turn makes the testing more robust (avoiding human error) while improving data integrity and simultaneously reducing QC costs.
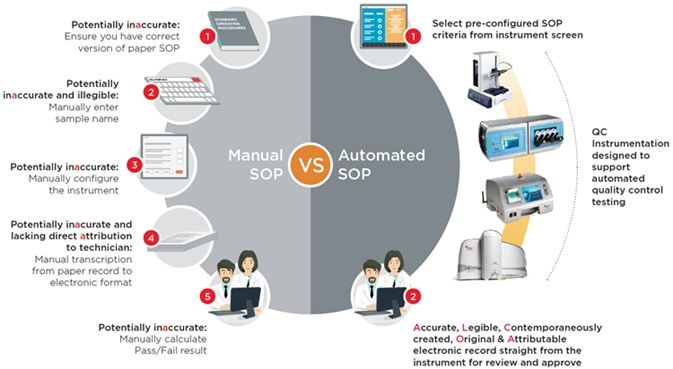
Image Credit: Beckman Coulter Life Sciences
Purified water and water for injection QC testing
The United States Pharmacopoeia defines TOC and conductivity as two of the four essential quality attributes defined for PW and WFI.11 It is possible to validate on-line analysis tools like the ANATEL PAT700 to be fully compliant with both of these vital quality attributes, and according to the pharmacopoeial requirements.
The FDA Process Analytical Technologies (PAT) initiative4 urged the pharmaceutical industry to invest in on-line process control equipment, instead of heavily relying on final product quality testing. This ensures better in-process quality control, as well as resulting in time and money savings.
Right-first-time quality for batch manufacturing is the most ideal outcome. Final product tests on pharmaceutical products are destructive, so 100% batch testing is not an option. At the same time however, one mistake during sampling and testing may see the whole batch facing destruction.
In order to mitigate these risks, large numbers of pharmaceutical manufacturers are connecting their on-line TOC analyzers to their factory control systems. This allows potential TOC or conductivity excursions to be detected and production halted, preventing potentially contaminated water from being mixed with valuable active pharmaceutical ingredients.
Revisions to the European Pharmacopoeia (EP) chapter on WFI now allow the production of WFI from ultra-filtration and double pass reverse osmosis (RO).12 With this in mind, TOC and conductivity monitoring of pharmaceutical water systems have become increasingly significant. This is due to the perceived risk of potential contamination break-through in the RO system, when compared to the more secure barrier afforded by a water still.
The 4 FDA water quality attributes for PW and WFI are:
- Inorganic
- Organic
- Endotoxins
- Microbial
Within the current guidance, the FDA emphasizes that the 21CFR part 11 ruling is only applicable to the data historian where electronic records are stored.10 The risk of on-line instruments with a built in, local data historian may need to meet the full requirements of the 21CFR part 11 ruling.
Tools like the PAT700 help to avoid this issue by enabling the local data historian to be disabled, therefore guaranteeing that it does not attract the full 21CFR part 11 requirement as a data archive used for electronic records.
Data from on-line TOC analyzers has been traditionally held in validated Distributed Control Systems (DCS) or Supervisory Control And Data Acquisition (SCADA) systems. This not only offers process control improvements, but it also adds a substantial amount of change control.
Contemporary approaches store quality-critical data records in their own secure archive, allowing SCADA and DCS systems to be more agile, and dedicated solely to process control.
To support this, it is possible configure the PAT700 to automatically send PDF electronic records for review and batch release, transmitting these via secure FTP over Ethernet. This process meets the ALCOA requirements for electronic records to be legible, contemporaneous, original and attributable.
While laboratory water quality testing instrumentation uses reagents, necessitating manual configuration for each sample along with regular (often daily) calibration, the on-line PAT700 does not suffer from this issue, requiring no reagents and offering automated test protocols. Additionally, the equipment only needs to be calibrated annually.
Calibration and System Suitability Testing SOPs are wholly automated, meaning there is no need for manual data entry. Additionally, pass/fail reports are automatically generated, removing the need for manual calculations.

Figure 1. Closed view of the ANATEL PAT700 Total Organic Carbon Analyzer. The ANATEL PAT700 exports PDF data straight to data archive via Ethernet. The PAT700 calibration standards export certified value, lot number and expiration date via RFID. Image Credit: Beckman Coulter Life Sciences
Cleanroom routine environmental monitoring
While GMP dictates the air quality conditions for cleanrooms used in biopharmaceutical production, the real risk comes from the microbes present on the human body. Human beings shed around 30,000 skin cells every hour,5 and all of these are potentially carriers of microbes. The technology does not yet exist to detect airborne microbes in real-time, so air particle counters are currently used as a substitute.

Figure 2. Humans and microbial contamination. Image Credit: Beckman Coulter Life Sciences
Discussions with Environmental Monitoring Managers at facilities worldwide highlight an increasing trend whereby the responsibility for environmental monitoring is being shifted from QC microbiology teams to production staff.
This is occurring for two main reasons: Firstly, microbiology staff are comparatively expensive to employ in carrying out such routine roles and secondly, it means less people inside the cleanrooms themselves, reducing any potential for product contamination. Challenges arise however, because the production team do not have the same knowledge of routine environmental monitoring.
In bigger biopharmaceutical manufacturing plants, teams of 10 or more technicians are responsible for thousands of routine environmental monitoring samples every month. At each sampling location, technicians must manually type the location name into the counter before sampling begins.
Furthermore, counters should be manually configured in line with written SOPs. At the end of every day, the hard copy print outs from each sample location are photocopied – this is because the printers in the particle counters are thermal, so these print-outs will fade over time. Finally, results from every location must be manually and individually converted into an electronic format.
Table 1. Manual cleanroom routine environmental monitoring SOPs. Source: Beckman Coulter Life Sciences
Touch-Point
1 |
Touch-Point
2 |
Touch-Point
3 |
Touch-Point
4 |
Touch-Point
5 |
- Ensure
correct SOP
- Read and understand
SOP
|
Manually type
each and every
sample location name |
Manually configure counter:
- Sample time
- Number of samples
- Results averaging
- Correct multiplier for m3
|
Take paper print-out
and photocopy |
Manually transcribe
results |
Some manufacturers have optimized their particle counting instrumentation specifically for pharmaceutical QC use, as part of their efforts to improve data integrity. This has included building in the capability for preconfigured electronic SOPs into the instrument design itself, enabling the generation of 21CFR part 11 electronic records directly from the instrument.
To achieve this, the user selects the preconfigured electronic SOP before pressing the ‘Start’ button. This prompts the instrument to accurately configure itself according to the SOP, before carrying out the appropriate test. Finally, an electronic test result record is automatically produced.
As well as enhancing data integrity, this automated approach has the potential to reduce costs as there is no requirement for technicians to create hard copy records and manually transfer this data into an electronic format.
Deskilling the use of air particle counters via automated SOPs allows the responsibility for cleanroom routine environmental monitoring to be shifted from qualified microbiologists to production staff, reducing costs even further.
Collapsing the routine environmental monitoring workflow with electronic SOPs
Table 2. Manual SOP. Source: Beckman Coulter Life Sciences
| Touch-Point 1 |
Touch-Point 2 |
Touch-Point 3 |
Touch-Point 4 |
Touch-Point 5 |
| Manual SOP |
Manually enter
location name |
Manually configure
counter |
Manually
transfer
data |
Review and
approve |
Table 3. Electronic SOP. Source: Beckman Coulter Life Sciences
| Touch-Point 1 |
|
Touch-Point 2 |
Select pre-configured
SOP from counter screen |

MET ONE 3400
Environmental Monitoring
Air Particle Counting
|
Review and approve
results in Excel, PDF or XML |
Viable cell counting
Cell therapy products, particularly homogeneous cell populations, are normally enumerated for viability and concentration based on the cells’ ability to exclude a supravital dye, such as Trypan Blue. This is generally accomplished manually via the use of a microscope and haemocytometer, but this method can be both prone to errors and laborious.
Initially designed for quantifying blood cells, the sample volume used in the haemocytometer method is generally only 100 nanolitres. Even minor errors using this method which are caused during sample dilution, pipetting, mixing or visual enumeration by the technician, may result in substantial errors in results. This is because the final count result is scaled up to report cell concentration in viable counts/mL.
One study undertaken of a team of 16 experienced, knowledgeable technicians using the haemocytometer method showed variances in reported cell concentration results of up to +/-20% between members.4
The USP chapter <1046>9 suggests that automated cell counting and viability instruments may offer a higher degree of accuracy alongside a more reproducible enumeration. These improvements are ideal if the sample preparation stage is automated, reducing any potential opportunities for technician errors.
Results are more likely to be reproducible and accurate where the automated cell counter is able to utilize electronic, pre-programmed cell-counting SOP libraries that match the manual, user-designed SOPs for cell counting.
Not only does this approach improve accuracy and reproducibility, automated cell counters may lower the cost of this QC test by enabling technicians to undertake other tasks while waiting for the counter to automatically complete the preparation and counting stages for each sample.
Counters which are capable of counting from 96-well plates also offer significant savings in terms of technician time, which results in a reduction of QC costs.
Automating the cell counting SOPs results in the method being more robust, generating the electronic record contemporaneously, leading to improved data integrity. Because of this, the results are attributable, legible, contemporaneously created, original and accurate, as per the FDA ALCOA guidance in the 21CFR part 11 guidance document.6
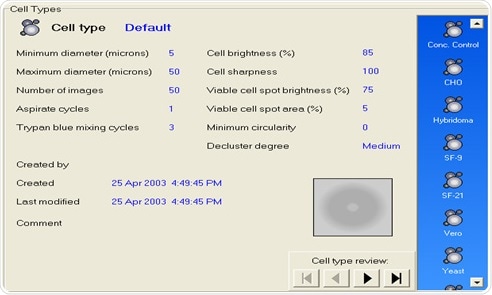
Figure 3. Automated Vi-CELL XR cell counting SOP library screen. Image Credit: Beckman Coulter Life Sciences
Injectable drug final product particulate testing
Though generally harmonized, requirements for parenteral drug particulate testing are occasionally varied between different countries and different products.
The sample volume to be analyzed and the results reporting format may vary considerably between products. For example, sampling requirements for small volume therapeutic protein products like vaccines7 are likely to be different to sampling requirements for a large volume parenteral like an intravenous drip bag.8
It is important that results are calculated and expressed in an appropriate format for the product under test, for example counts per container or counts per mL.
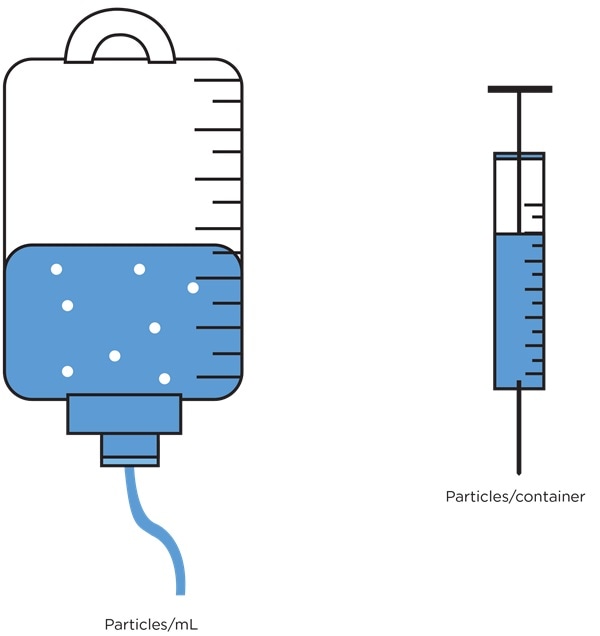
Figure 4. Particulate contamination reporting requirements are product dependent. Image Credit: Beckman Coulter Life Sciences
While general-purpose liquid particle counting equipment may be suitable for the testing of particles in parenteral products, counters which are optimized for the specific application are a better option, primarily due to the varying degrees of complexity involved in the testing.
Particle counters which are suitably optimized for this testing incorporate various compendial tests, being able to calculate a pass/fail result automatically. Because QC teams will often use a product brand name to label the product sample under test, optimized particle counters simply involve the user selecting the necessary test for each sample by choosing the appropriate product by name from a drop-down menu.
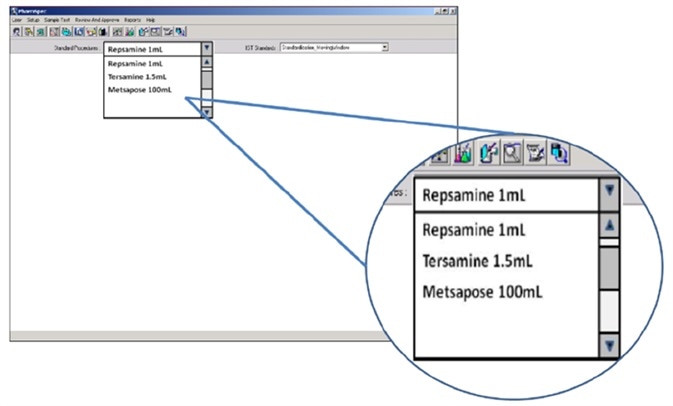
Figure 5. Electronic SOP functionality in the HIAC PharmSpec software allows automated SOP test routines to be named according to the brand name of the product under test and selected by the technician via drop-down box. Image Credit: Beckman Coulter Life Sciences
Conclusion
While the industry focuses on omissions and errors in 21CFR part 11 electronic records, the fundamental issue is that retraining may not be the whole solution. Manual SOPs are simply not robust enough to prevent recurrences of data errors and omissions in the future.
QC instrumentation which automates the quality control SOP has the potential to reduce the impact of human error on data integrity issues while simultaneously reducing the time that technicians must spend carrying out the SOP, both resulting in important cost savings.
References
- Managing the Cost of Compliance in Pharmaceutical Operations, Frances Bruttin and Dr. Doug Dean, IBM Business Consulting Services, Pharmaceutical Sector, Aeschenplatz 2, CH-4002 Basel, Switzerland, April 2004
- An Analysis of FDA Warning Letters, Barbara Unger, Unger Consulting Inc., Pharmaceutical Online Guest Column, July 14, 2017
- Guidance for Industry: PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA, September 2004
- Comparison of Manual versus Automated Trypan Blue Dye Exclusion Method for Cell Counting, Kristine S. Louis, Andre C. Siegel, Gary A. Levy, Industrial BioDevelopment Laboratory.
- Health How Stuff Works, How many skin cells do you shed every day?, by Ed Grabianowski. http://health.howstuffworks.com/skin-care/information/anatomy/shed-skin-cells.htm, published July 6, 2010
- Data Integrity and Compliance With CGMP, Guidance for Industry, April 2016, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA.
- USP<787> Subvisible Particulate Matter In Therapeutic Proteins, Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA
- USP<788> Particulate Matter in Injections, Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA
- USP<1046> Cell and Gene Therapy Products – Analytical Methodologies, Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA
- Guidance for Industry, Part 11, Electronic Records; Electronic Signatures — Scope and Application August 2003 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA
- United States Pharmacopoeia U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA
- European Pharmacopeia (Ph. Eur.) Commission press release 18th March 2016 .EDQM Expert Workshop, 24 March 2011 European Directorate for the Quality of Medicines & Healthcare (EDQM) 7 allée Kastner, CS 30026 F -67081, Strasbourg
About Beckman Coulter Life Sciences
Beckman Coulter Life Sciences is dedicated to empowering discovery and scientific breakthroughs. The company’s global leadership and world-class service and support delivers sophisticated instrument systems, reagents and services to life science researchers in academic and commercial laboratories, enabling new discoveries in biology-based research and development.
A leader in centrifugation and flow cytometry, Beckman Coulter has long been an innovator in particle characterization and laboratory automation, and its products are used at the forefront of important areas of investigation, including genomics and proteomics.
Primary activity / Product lines
- Flow Cytometry
- Centrifugation
- Particle Counting and Characterization
- Liquid Handling and Robotics
- Nucleic Acid Sample Preparation
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.