The FDA emphasizes the importance of corrective and preventive action (CAPA) for any deviations potentially impacting product quality In their 21CFR guidance.
Regulatory authorities expect manufacturers to examine deviations, take corrective action, put in place interventions to stop any recurrence of the deviation and to assess the intervention’s effectiveness. The success of those interventions is questionable without adequate root-cause investigation.
Yet, TOC or conductivity contamination events can be transient in nature. For instance, TOC excursions could occur in the distribution loop in a water system which is experiencing gradual biofilm build up just after sanitization cycles as biofilm sloughs off the inside of the pipework.
By being oxidized and broken down by UV lamps connected to the distribution network put in place to discourage microbial build-up, or by being diluted as the water passes into the large quantity of water in the storage tank, this excursion in the distribution loop can disappear quickly.
As it is not easy to get a sample of the excursion as there is not enough time to do so, this transient nature makes root-cause investigations challenging. Yet, this quick disappearance may mask the increasing biofilm build up until there is a breakdown of system control and run-away microbial contamination.
In this article some typical sources of contamination in water systems are outlined and how root-cause investigations can be supported by utilizing online Total Organic Carbon (TOC) analyzers.
FDA water quality expectations
The employment of the terms Water For Injection (WFI) and Purified Water (PW) implies that these waters adhere to the quality expectations defined by the pharmacopoeias. There are four vital quality attributes that define PW and WFI:
- Endotoxins – Pyrogenic substance found in the cell wall of gram-negative bacteria that can cause a fever if injected into patients
- Conductivity – Inorganic contaminants which could cause illness in patients and are not meant to be present
- Microbial colony forming units (CFU) – Potentially harmful bacteria which could cause infection and illness in patients
- Total organic carbon (TOC) – Organic material which could encourage microbial growth in water systems
At present, only TOC and conductivity can be measured online in real time. The CFU and endotoxin tests are both laboratory-based tests.
The EDQM Expert Workshop2 recommended a focus on TOC and conductivity as early indicators of potential microbial contamination or increase in microbial by-products, which could be endotoxins, in their report prepared for the European Directorate.
Water system TOC or conductivity deviations could indicate potential endotoxin or microbial excursions and should be examined, but these events are often transient in nature and the excursion has disappeared by the time grab samples are taken to help the root-cause investigation.
Viable non-culturable microbes
Microbes which are viable but non-culturable could be said to be incapable of proliferation and considered sterilized. Yet, microbes which are no-longer living can be said to be sanitized, i.e. without life.
Whilst microbes can still exist in the biofilm that builds up over time in water systems5,heated water systems may keep microbes from proliferating, i.e. sterilized, which is why heat sterilization should be repeated on a regular basis. If it truly was a sanitized environment, i.e. absent of life, then the heating process would not need repeating.
Examples of sources of contamination
TOC from solvent in bulk active pharmaceutical ingredient (API) plant
The author discovered one root-cause investigation in a bulk API manufacturing facility which led the team to consider contamination from one of the organic solvents that was utilized in the API manufacturing process, but how did it get into the WFI water system?
A physical inspection of the location of the WFI storage tank demonstrated that there was a production waste drainage cover near to the tank, but still how was the solvent getting into the tank?
The team was able to establish that the excursions closely followed the end of a production run when the production vessels were being cleaned down ready for the next batch, by studying the pattern of TOC excursions.
So, a unique combination of events were occurring at the same time: the waste containing the solvent was passing down the drain and, being a volatile substance, some of the solvent formed a vapour above the drain, whilst at the same time there was a large demand for WFI from the storage tank to complete the cleaning of the production vessels.
Quickly dropping water levels inside the WFI tank resulted in air being drawn into the tank through the vent, which was located over the drain cover. Solvent vapour was clearly being drawn into the tank along with the air.
Re-siting the WFI storage tank vent away from the drain cover supplied a permanent solution, stopping recurrence of the contamination event.
TOC from point of use
Although water systems are pressurized, it has been known for a syphoning effect to happen when a point of use is first opened.
It is common practice to wipe the point of use down with industrial alcohol (IPA) when gathering grab samples for microbial and endotoxin testing in the laboratory, this is to prevent any potential microbial contamination of the sample by microbes present on the outside of the point of use.
Yet, this alcohol is an organic material and this could result in a TOC excursion, should some be syphoned into the point of use.
TOC from autumn leaf-fall
The levels of organic material in source water can also differ by season. High levels of leaf-fall in autumn can heighten organic content of feed waters, which then become a challenge to PW make-up treatment plants.
Treatment plants are designed to take away some of the incoming water contaminants, but higher contamination levels in the incoming water may lead to increases in contamination levels in the PW system.
False TOC results
To oxidize the organic molecules and release the carbon in the form of carbon dioxide, all pharmaceutical-grade TOC analyzers employ UV light. Through sensor drift in TOC analyzers using two sensors to measure Total Carbon (TC) and Total Inorganic Carbon (TIC), and then calculating TOC, TOC results can be reported higher than they actually are.
Similarly, ageing of the UV lamps will gradually influence the intensity of the UV light generated. This drop-off in intensity may lead to under-reported TOC values in constant-flow TOC analyzers where the sample has a fixed exposure time to the UV light, which would stay undetected until the UV lamp was replaced.
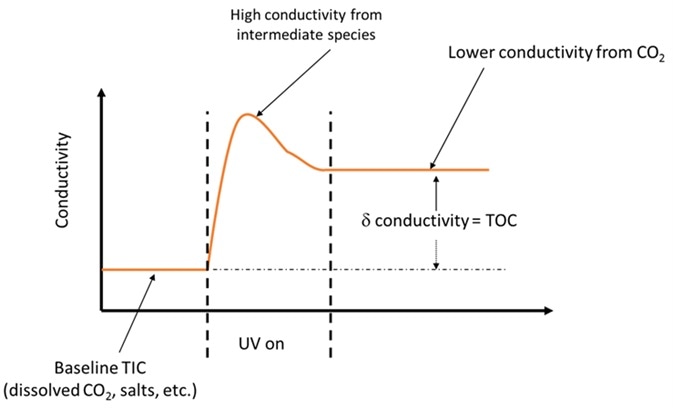
Figure 1. Intermediate species created during partial oxidation can be the cause of false TOC results. Image Credit: Beckman Coulter Life Sciences
The EP 2.2.44 chapter on TOC analysis emphasizes how important complete oxidation of the organic contamination is in order to get an accurate TOC measurement4. Longer chain, more complex organic molecules may produce organic acids in the process of being oxidized down to CO2.
Organic acids may contribute much more to the conductivity measurement than the final CO2 from the completely oxidized organic, so failure to ensure complete oxidation of the organic may lead to an incorrect TOC value.
TOC from RO integrity breach
In March of 2016, the chapter for WFI in the European Pharmacopeia was altered to enable, for the first time, the production of WFI using reverse osmosis (RO)1.
The EDQM Expert Workshop2 warned that reverse osmosis systems may not be as robust as stills and recommended a focus on on-line TOC and conductivity as early indicators of an increase in microbial by-products which could be endotoxins, or potential microbial contamination, in their report prepared for the European Directorate.
Concerns were voiced that microbial build-up on the feed water side of the RO could potentially break through the RO membrane and contaminate the water system.
In the same way, microbial by-products like endotoxins could pass through the RO membranes. These would be picked up as an increase in TOC levels and possibly also as an alteration in oxidation profile.
Support for root-cause investigations
Avoiding false TOC results
UV light is the main technique utilized by most pharmaceutical-grade TOC analyzers to oxidize the TOC. Almost all TOC analyzers which utilize UV light only have a single UV lamp and do not monitor the level of UV light the lamp emits, potentially ignoring a decrease in UV light output which could compromise the ability of the analyzer to measure TOC.
The typical lifetime of these UV lamps is approximately one year, the analyzer will no-longer be able to analyze TOC if the UV lamp fails completely. The level of TOC can be <10ppb in modern WFI systems and a failed TOC analysis UV lamp may go unnoticed, leaving potential TOC excursions in the WFI system to pass undetected.
If a failure of the UV lamp is detected by the user, it could prompt the requirement for increased grab sample TOC analysis by the QC laboratory until the TOC analyzer can be repaired.
The PAT700 has main and a standby UV light and monitors the UV light output. The standby lamp is automatically activated and an alarm is set to notify the user if the UV light from the main lamp falls under an acceptable level for good TOC analysis.
Figure 2. The PAT700 protects against unplanned down-time and expensive service call-outs by having auto-switching main and stand-by UV lamps. Image Credit: Beckman Coulter Life Sciences
To completely turn all of the carbon present to carbon dioxide for accurate TOC measurement, different organic materials may need different oxidation times, the PAT700 monitors the change in the conductivity measurement and waits until there is no more change in order to detect that the organic has been completely oxidized, ensuring accurate TOC analysis.
It also has only one measurement cell to measure TIC and TC, so sensor drift will not influence the accuracy of the calculated TOC.
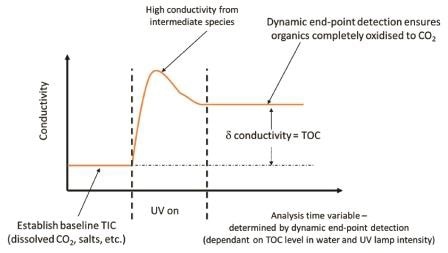
Figure 3. PAT700 uses dynamic end-point detection to ensure complete oxidation for accurate TOC analysis, even when UV lamp intensity decreases. Image Credit: Beckman Coulter Life Sciences
Excursion capture
The PAT700 may be programmed to gather a water sample the instant that a TOC excursion is detected so that the sample can be analyzed further to discover the root cause.
Figure 4. The PAT700 can capture a water sample to support root cause analysis if a TOC excursion is detected. Image Credit: Beckman Coulter Life Sciences
Calibration best practices
It is vital to make sure that any reported deviations in TOC or conductivity levels are a real deviation and not in fact caused by a drift in the calibration of the analyzers. So the first step in any root cause investigation after a deviation is to verify that the TOC or conductivity analyzer is still measuring accurately.
The majority of pharmaceutical manufacturers usually have their TOC analyzers calibrated every six months. There are two types of calibration; calibration validation and calibration adjustment.
In calibration validation, the performance of the TOC analyzer is compared to the certified values after certified calibration standards are run as grab samples. If the reported values from the TOC analyzer are within an acceptable percentage of the certified values of the standards (blank subtracted) a ‘Pass’ is given.
Certified calibration standards are run through the TOC analyzer for calibration adjustment. The TOC analyzer modifies its calibration slope to give the best fit against the certified values of the standards.
The new calibration is considered acceptable and a ‘Pass’ is given as long as the TOC analyzer can modify its slope to give a correlation coefficient (linearity factor) ≥0.990 and as long as the change in the calibration slope of the TOC analyzer is not a large change from the original factory calibration.
A modification in the calibration slope which deviates a lot from the factory slope is considered a sign that something has gone wrong, i.e. either the analyzer is working incorrectly, or the calibration standards are not matching their certified value.
Although it is not mandated, it is thought to be best practice to perform the following step-by-step procedure when calibrating a TOC analyzer:
- Perform an ‘as found’ calibration validation prior to undertaking any maintenance or adjustments. This verifies that the TOC analyzer has been working within specification since the last calibration and that nothing has gone wrong since then.
- Perform a calibration adjustment. This ‘fine tunes’ the calibration of the TOC analyzer to make it as accurate as possible and protects against gradual drift in calibration over longer periods of time.
- Perform an ‘as left’ calibration validation. This verifies that the calibration slope adjustment carried out in Step 2) has led to an accurate calibration compared to traceable, certified standards
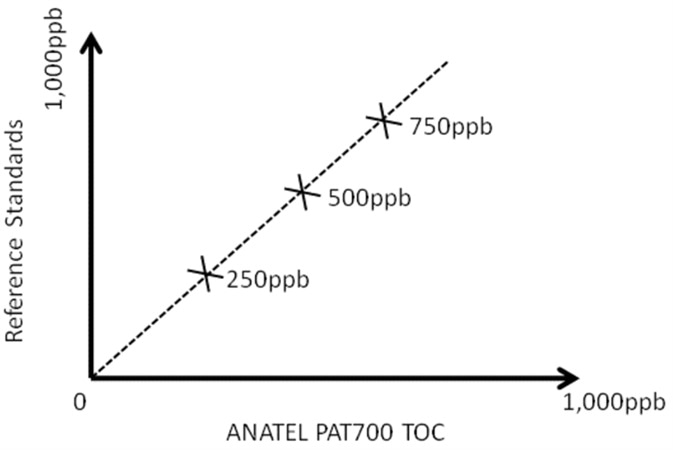
Figure 5. 'As found' calibration carried out before any maintenance procedures to confirm TOC analyzer is still performing acceptably after 6 month's use. Image Credit: Beckman Coulter Life Sciences
System suitability
This test is designed to ensure that the TOC analyzer can analyze the range of organic contaminants that could happen in PW and WFI equally. With the concerns around potential microbial contamination or from microbial by-products this becomes more significant, as the type of organic contamination may change with time.
Conductivity calibration
The drawback of traditional conductivity instruments is that the measurement cell is inside the WFI pipe and it is inaccessible, so the cell constant is unable to be checked as recommended in the United States and European pharmacopoeias (USP and EP)3,4 by utilizing a conductivity solution of a known, certified value.
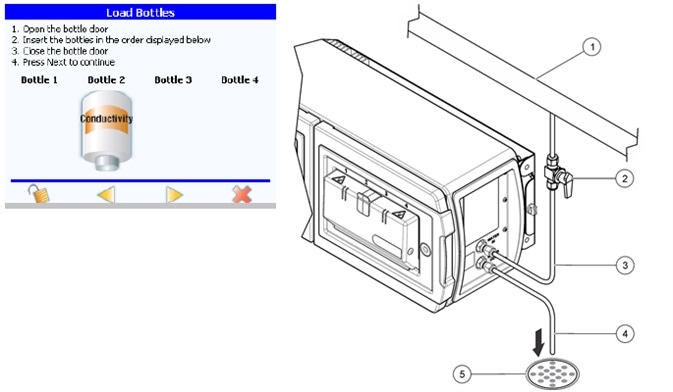
Figure 6. The PAT700 direct conductivity analyzer cell constant can be verified according to the requirements in USP <645>. Image Credit: Beckman Coulter Life Sciences
TOC analyzers that use direct conductivity measurement as part of their TOC analysis like the PAT700 from Beckman Coulter, can have the cell constant checked as the sample entering the conductivity cell subsequently goes to drain, and so, does not compromise the water loop itself.
The majority of TOC analyzers are unable to comply with the USP and EP requirements to verify the accuracy of the conductivity meter electronics with an external resistor. The PAT700 has been specifically designed to support this need, thus satisfying all the compliance requirements of the USP and EP as a conductivity analyzer.
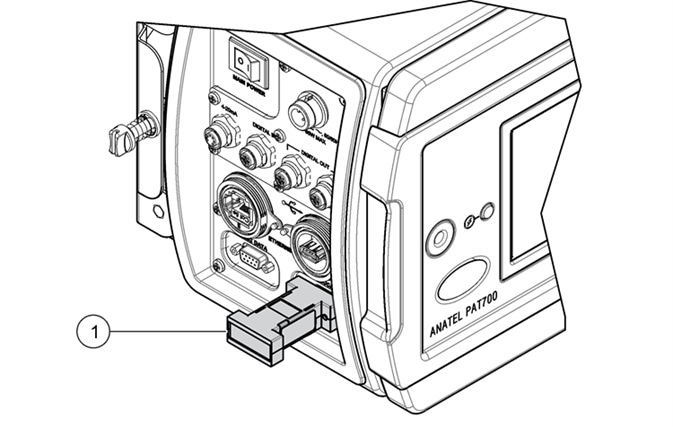
Figure 7. PAT700 direct conductivity analyzer meter accuracy can be verified using external resistor as per the requirements of USP<645>. Item 1 indicated in the illustration represents the external calibration resistor. Image Credit: Beckman Coulter Life Sciences
Detecting alterations in water organic chemistry
A change in the oxidation profile may signal a change in water organic chemistry, as different organic materials can generate different TOC oxidation profiles. In order to ensure accurate TOC analysis, the European Pharmacopoeia emphasizes the need for complete oxidation of the organic contamination4.
Combined with a change to the oxidation profile, heightening TOC levels could be a sign of a change in the organic chemistry of the water and should be explored before a potentially larger excursion happens. Some TOC analyzers, like the PAT700 from Beckman Coulter, give an indication of the oxidation curve profile during each TOC analysis.
Combined with a change in TOC levels, a change in the type of profile may give insight into a potential degradation of the RO system integrity, encouraging the user to further investigate and consider corrective action to stop a large-scale contamination event and to put in place preventative measures to avoid the recurrence of the problem in the future.
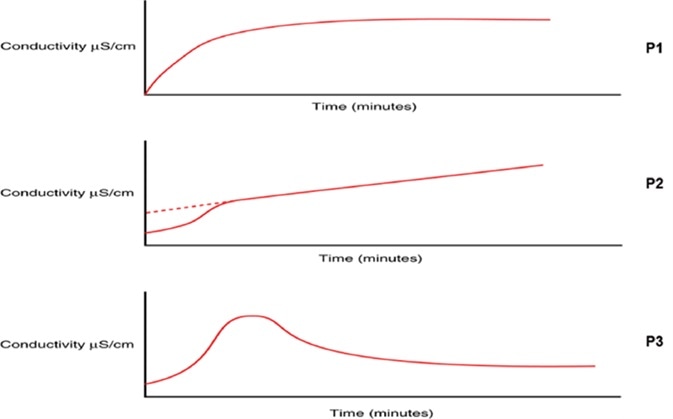
Figure 8. Changes in TOC oxidation profile curve can indicate potential degradation of water treatment integrity, prompting investigation to prevent a large-scale contamination event. Image Credit: Beckman Coulter Life Sciences
Grab sample analysis
Grab samples should be taken at different points on the loop as part of the root cause investigation, in order to identify if the contamination is coming from one of the points of use on the water loop.
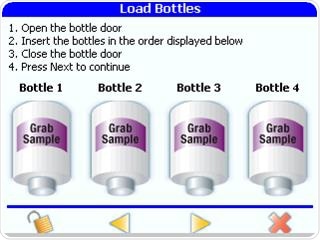
Figure 9. Analyzing point of use grab samples to help with root cause investigations. Image Credit: Beckman Coulter Life Sciences
The PAT700 from Beckman Coulter enables users to analyze up to four grab-samples at a time by utilizing the on-board auto-sampler. As they are generated in secure, password protected PDF files including the technician’s electronic signature, 21CFR part 11 compliance is permitted for the results.
Conclusion
As PW or WFI contamination events can be transient in nature, TOC analyzers that can capture a sample of the contaminated water can supply valuable assistance in root-cause investigations.
Data on alterations to the oxidation profiles from the TOC analyzer can be a useful indicator of potential changes to water organic chemistry, giving an early warning of potential deterioration in RO membranes.
Similarly, avoiding false high or low TOC results with robust analyzer design can help decrease wasted time in unneeded root-cause investigations and avoid the ‘no fault found’ results which are unwanted by regulators. A well considered choice of TOC analyzer can give much needed support for water system deviation root-cause investigations.
References
- European Pharmacopeia (Ph. Eur.) Commission press release 18th March 2016 https://www.edqm.eu/en
- EDQM Expert Workshop, 24 March 2011 European Directorate for the Quality of Medicines & Healthcare (EDQM) 7 allée Kastner, CS 30026 F -67081 Strasbourg
- US Pharmacopeia Convention, United States Pharmacopoeia, Rockville MD, USA and Council of Europe, European Directorate for the Quality of Medicines & Healthcare, European Pharmacopoeia, Strasbourg, France
- Council of Europe, European Directorate for the Quality of Medicines & Healthcare, European Pharmacopoeia 8.0, 01/2008:20244, Total Organic Carbon in Water for Pharmaceutical Use, Strasbourg, France
- International Society for Pharmaceutical Engineering, The ISPE Good Practice Guide: Ozone Sanitization of Pharmaceutical Water Systems, First edition July 2012 https://guidance-docs.ispe.org/Good-Practice-Guide-Ozone-Sanitization-of-Pharma-Water-Systems [14th August 2014]
- Pharmaceutical and Healthcare Sciences Society, Best Practice for Particle Monitoring in Pharmaceutical Facilities,
- PHSS Technical Monograph No.16, First Edition 2008, ISBN 978-1-905271-15-3
About Beckman Coulter Life Sciences
Beckman Coulter Life Sciences is dedicated to empowering discovery and scientific breakthroughs. The company’s global leadership and world-class service and support delivers sophisticated instrument systems, reagents and services to life science researchers in academic and commercial laboratories, enabling new discoveries in biology-based research and development.
A leader in centrifugation and flow cytometry, Beckman Coulter has long been an innovator in particle characterization and laboratory automation, and its products are used at the forefront of important areas of investigation, including genomics and proteomics.
Primary activity / Product lines
- Flow Cytometry
- Centrifugation
- Particle Counting and Characterization
- Liquid Handling and Robotics
- Nucleic Acid Sample Preparation
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.