One of the quality characteristics defined in the European and USA pharmacopeias for pharmaceutical waters is Total Organic Carbon (TOC)1. Contemporary water treatment systems are able to provide water with such high purity that TOC levels can be consistently near zero and very hard to measure with any accuracy.
This article explains a few of the difficulties faced when using TOC analyzers to validate pharmacopoeial TOC level compliance for contemporary water systems with consideration of the ICH Q2 document2 from the International Conference on Harmonisation.
ICH Q2
The International Conference on Harmonisation underlines features for consideration during the validation of the analytical procedures in their ICH Q2 guidance document, Validation of Analytical Procedures2.
It consists of terms and definitions with the aim of bridging the differences that frequently occur between a variety of compendia and regulators of the EC, Japan and the USA. Individuals who use TOC analyzers to measure the impurities existing in pharmaceutical-grade waters might find the advice and guidance helpful.

Image 1. The QbD1200 TOC Analyzer. Image Credit: Beckman Coulter
TOC analysis
TOC analysis in pharmaceutical-grade waters is a non-specific test in that it effectively measures the weight in parts per billion (ppb) of carbon that is a resultant from organic material in the water but is unable to differentiate from different types of organic material.
Additionally, it is unable to report the definitive amount of organic material present because the quantity of carbon in an organic molecule differs between varying organic materials. For instance, a sucrose molecule consists of 12 carbon atoms, whereas a methanol molecule consists of only one carbon atom.
If a TOC analyzer reports 100 ppb TOC, it could mean that the water consists of a large number of molecules of an organic material that has a very low number of carbon atoms present, or it is possible that there is a much smaller amount of molecules consisting of a larger amount of carbon atoms per molecule.
| Common terms describing carbon in water |
| Total Organic Carbon |
TOC |
| Total Carbon |
TC |
| Total Inorganic Carbon |
TIC |
Figure 1. Common terms describing carbon in water. Source: Beckman Coulter
Measurement accuracy
All TOC analyzers in frequent use for pharmaceutical water systems have the same aim of oxidizing the organic material existing in the water3 and then determining the consequential carbon dioxide released from the oxidized organic molecule.
This carbon dioxide is measured in gas phase by some analyzers, while others measure in dissolved phase. Numerous approaches are utilized to oxidize the organic, and the three most common types used in the pharmaceutical industry are ultra-violet (UV) light exposure, persulphate in the presence of UV light and high-temperature combustion.
Measurement accuracy is discussed in ICH Q2 and it proposes that accuracy could be incidental after the establishment of precision, linearity and specificity. It is also suggested that linearity is determined using a minimum of 5 concentrations of the traceable standard.
The Joint Committee for Guides in Metrology propose in their Guide to the expression of uncertainty in measurement4 that the more complex a measurement is, the higher the uncertainties of the measurement. This is due simply to the larger quantity of estimations and assumptions within the measurement technique, thus influencing the accuracy and the ability of the analyzer to measure very low levels of analyte.
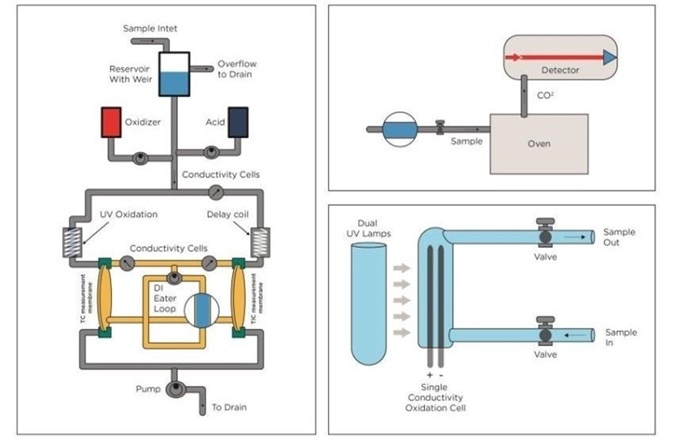
Figure 2. TOC technologies. From left: UV Persulphate combined with membrane conductometric, High Temperature Combustion and UV only. Image Credit: Beckman Coulter
Specificity challenges
In their ICH Q2 guidance document Validation of Analytical Procedures2, The International Conference on Harmonisation emphasizes the requirement for an analytical procedure to have specificity, for example, “the ability to assess unequivocally the analyte in the presence of components which may be expected to be present”3.
One of the main specificity difficulties in measuring the carbon dioxide from organic material in water is that pharmaceutical waters also consist of moderately large quantities of total inorganic carbon (TIC) in the form of carbonates and dissolved carbon dioxide gas. This is mostly due to the higher concentration of dissolved carbon dioxide gas in the water as a result of the reverse-osmosis process (RO) used to manufacture pharmaceutical water.
Therefore, it can be difficult when attempting to measure very low TOC water when there is a large quantity of TIC present, particularly in TOC analyzers which utilized numerous sensors to measure TC and TIC and then minus one from the other to work out the TOC content of the water. This is seen in Figure 3.
TOC = TC - TIC
Figure 3. Calculating TOC from Total Carbon (TC) and Total Inorganic Carbon (TIC).
Analyzers that depend on working out the TOC from TC and TIC face a difficulty when wanting to measure very low quantities of TOC when fairly high quantities of TIC are present because moderately small inaccuracies between the TIC and TC sensors can result in either over- or under-reporting of TOC5 This can be seen Figure 4.
| . |
| Total Organic Carbon (TC) |
2,000 ppb |
| Total Inorganic Carbon (TIC) present |
1,900 ppb |
| Actual TOC present |
100 ppb |
| Analyzer measurement accuracy |
2% |
| Analyzer measured TC |
Between 1,960 and 2,040 ppb |
| Analyzer measured TC |
Between 1,862 and 1,938 ppb |
| Analyzer calculated TOC |
Between 22 and 178 ppb |
Figure 4. Example showing that TOC analyzers that use multiple sensors to measure TC and TIC and then calculate TOC can suffer from measurement inaccuracies5. Source: Beckman Coulter
It can be very hard to feel assured that the pharmaceutical water is of suitable quality for production when the characteristic measurement doubts in the TOC analyzer can result in a possible inaccuracy in reported TOC level of +/-78%. This is based on the example in Figure 4.
The issue is compounded for quality control laboratories who want to determine TOC in their water supply that is incoming. Seasonal differences in TIC levels will result in the user having to invest in a kind of TIC elimination device and always checking on the levels of TIC in their incoming water to ensure that it does not surpass the maximum level suggested by their TOC analyzer manufacturer.
Several analyzer manufacturers recommend a maximum ratio of TIC to TOC of 10:16. Therefore, in a water sample consisting of 10 ppb TOC, the TIC must not surpass 100 ppb for that analyzer to correctly work.
Usually, high-temperature combustion analyzers attempt to solve the issue of TIC by implementing a TIC removal step. The water sample pH is moved by the action of adding an acid, forcing the TIC to be removed from the solution as a carbon dioxide gas.
Then, the carbon dioxide from the TIC is sparged out of solution via bubbling of a CO2-free carrier gas through the sample. These sparging cycles are usually of a specific duration and there is a risk that all of the TIC may not be eliminated and some may stay and affect the TOC analysis. Therefore, users need to measure TIC levels in the water sample to make sure that they do not surpass the maximum levels detailed by their TOC analyzer manufacturer.
Alternatively, monitoring the TIC removal could be an option to make sure that the TIC is totally eliminated prior to starting TOC analysis. This technique steers clear of the TIC specificity difficulties and TOC measurement accuracy is independent of the levels of TIC present.
The process can be enhanced further by utilizing a single CO2 sensor to determine both the TIC and the TOC. Rather than calculating TOC from TC and TIC, this approach directly measures the CO2 from the TOC in an independent measurement after all of the TIC is totally eliminated.
The measurement sensor accuracy of +/- 2% is now associated only with the measured TOC value rather than the measured TC and TIC values utilized by the alternative approaches. Therefore, using the example in Figure 4 where the actual TOC value is 100 ppb, this approach would give a report of between 98 ppb and 102 ppb.
This provides far more confidence to the user that the stated TOC results accurately mirror the actual amount of TOC in the water from a direct measurement, instead of a calculation. This alternative approach, of course, depends on the ability of the analyzer to measure the total removal of the TIC. The sensor needs to have the ability to measure when the CO2 from the TIC has been eradicated prior to the ultraviolet light being turned on and oxidation of the organics to CO2 starts.
The detection limit challenge
The ICH Q2 guidance document distinguishes between three analytical methods: Identification, Testing for Impurities and Assay. While the document proposes that the quantitation limit of an analyzer may not be relevant in an impurity limit test, like the TOC test, it does say that the detection limit is an essential feature for such tests.
As mentioned previously in this paper, TOC analyzers state the weight in parts per billion (ppb) of carbon derived from organic material in the water. This comes with its own challenge as contemporary pharmaceutical water systems may consist of more than 10 ppb TOC and several laboratory TOC analysis technologies will find it hard to report accurately at these low levels.
Therefore, the analyzer is unable to report the level of TOC and the user is presented with error messages like “TOC level is below the limit of detection”. Needless to say, several users do not recognize this because the action of completing a grab sample from a water system will inevitably contaminate the sample resulting in TOC reports usually in excess of 100 ppb. Thus, those who own very low TOC water systems may actually be measuring and reporting the TOC contamination from the grab sampling process, not the TOC in their water system.
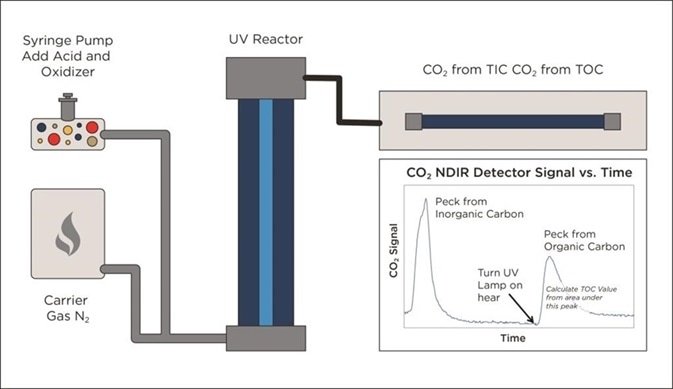
Figure 5. Alternative UV/Persulphate design monitors TIC removal before starting TOC analysis. Image Credit: Beckman Coulter
Very low TOC levels are even more challenging for analyzers that employ multiple sensors and estimate TOC by subtracting measured TIC from TC. The analyzer may actually report an estimated TOC value, even when the inherent accuracy errors in the multiple sensors used to measure the TC and TIC can have such a large impact on the accuracy of the reported TOC value5, as shown in Figure 4.
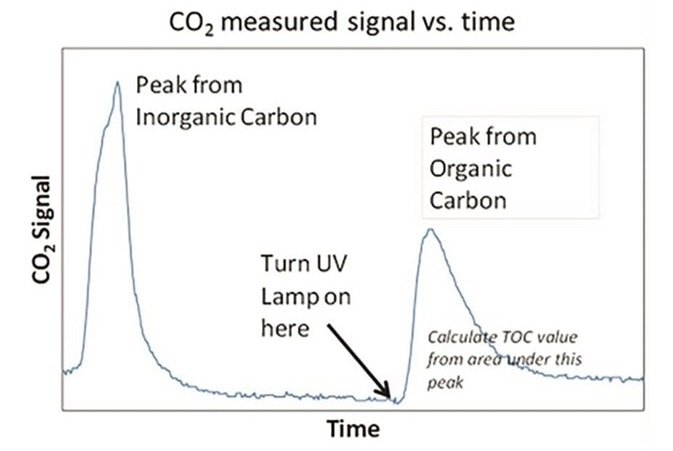
Figure 6. CO2 measured signal versus time. Image Credit: Beckman Coulter
While there is understanding that many analyzers are unable to resolve down to these low levels of TOC accurately and will only report “TOC level is below the limit of detection”, it is not comfortable to offer a batch of product using an absence of data when there is potential that the analyzer actually failed to take an accurate measurement because there was a lack of carrier gas or oxidizing reagent.
Users need to ensure the presence of the carrier gas and reagents prior to and post-analysis. This is usually completed by adding certified 500 ppb TOC standards into the batch of water samples to be analyzed at the beginning, middle and end of the analyzer’s autosampler tray.
However, as laboratory TOC analyzers are often prepared and utilized overnight, a carrier gas, or reagent supply failure during the night can result in the user becoming aware that the results from the batch of water samples are incorrect when they examine the analyzer the next day.
However, a re-test of the batch of water samples is not possible because the analyzer has used all the samples up when attempting to analyze them during the night. This can result in the user being left with no proof that the water system was complying during the batch of products manufactured the day prior.
Conclusion
Accurate total organic carbon analysis of low TOC contemporary pharmaceutical grade water faces several challenges. Instruments with numerous sensors that determine TC and TIC and then calculate the TOC can suffer from imprecise results because of TC and TIC measurement inaccuracies5. Analyzers with only one sensor to complete the measurements can provide an increasingly accurate result due to fewer estimations and assumptions in the measurement4.
Specificity in the presence of inorganic carbon is a difficulty for numerous analyzers. A method with higher accuracy involves removing the TIC and monitoring to ensure it has been totally removed prior to measuring the TOC directly.
Several analyzer designs are just unable to measure low ppb TOC levels because of the analyzers’ limit of detection due to the high number of estimations and assumptions in the measurement4. Though the pharmacopeias need the TOC analyzer to have a limit of detection of 50 ppb1, this is just insufficient when measuring water from a contemporary, low ppb TOC system.
Users wanting to utilize a broader ranging TOC analyzer that uses a mixture of oxidizing reagents and/or carrier gases should implement procedures to make sure that the analyzer is unable to continue to complete analyses and destroy water samples if the reagent or the gas are depleted.
This could be in the form of a manual check, or could be designed within the analyzer so that it constantly monitors all of the critical analysis parameters and ceases attempting to complete analyses should any issue arise.
The guidance given in the ICH Q22 guidance document can be useful for users to establish the suitability of the design and performance of laboratory TOC analyzers in the light of the difficulties of measuring TOC in contemporary low-TOC water systems discussed in this paper.
References
- US Pharmacopeia Convention, United States Pharmacopoeia, Rockville MD, USA and Council of Europe, European Directorate for the Quality of Medicines & Healthcare, European Pharmacopoeia, Strasbourg, France
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Validation Of Analytical Procedures: Text And Methodology Q2(R1), November 2005 [8th August 2014], http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf [8th August 2014]
- Council of Europe, European Directorate for the Quality of Medicines & Healthcare, European Pharmacopoeia 8.0, 01/2008:20244, Total Organic Carbon in Water for Pharmaceutical Use, Strasbourg, France
- Joint Committee for Guides in Metrology, Evaluation of measurement data — Guide to the expression of uncertainty in measurement ref. JCGM 100:2008 First edition September 2008, http://www.iso.org/sites/JCGM/GUM-JCGM100.htm [8th august 2014]
- GE Analytical Instruments, Technical Bulletin The Sievers Inorganic Carbon Remover (ICR), Boulder, Colorado, USA www.geinstruments.com [8th August 2014]
- GE Analytical Instruments, Sievers 900 Series Total Organic Carbon Analyzers, Operation and Maintenance Manual, Ref. DLM 90688-03 Rev. A, 2011, Boulder, Colorado, USA www.geinstruments.com [8th August 2014]
- International Society for Pharmaceutical Engineering, The ISPE Good Practice Guide: Ozone Sanitization of Pharmaceutical Water Systems, First edition July 2012 http://www.ispe.org/ispe-good-practice-guides/ozone-sanitization-pharmaceutical-water-systems [14th August 2014]
- Pharmaceutical and Healthcare Sciences Society, Best Practice for Particle Monitoring in Pharmaceutical Facilities, PHSS Technical Monograph No.16, First Edition 2008, ISBN 978-1-905271-15-3
About Beckman Coulter Life Sciences
Beckman Coulter Life Sciences is dedicated to empowering discovery and scientific breakthroughs. The company’s global leadership and world-class service and support delivers sophisticated instrument systems, reagents and services to life science researchers in academic and commercial laboratories, enabling new discoveries in biology-based research and development.
A leader in centrifugation and flow cytometry, Beckman Coulter has long been an innovator in particle characterization and laboratory automation, and its products are used at the forefront of important areas of investigation, including genomics and proteomics.
Primary activity / Product lines
- Flow Cytometry
- Centrifugation
- Particle Counting and Characterization
- Liquid Handling and Robotics
- Nucleic Acid Sample Preparation
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.