As part of the ISO periodic review cycle, ISO 14644-1, Cleanrooms and associated controlled environments — Part 1: Classification of air cleanliness is being revised. ISO TC209 Working Group 01, an international team of subject matter experts, is planning to assess the existing ISO 14644 document and propose changes for enhancements.
After discussing with statisticians, the expert working group is recommending modifications to the classification method. It is also implementing the key elements of ISO 14644-3, Cleanrooms and associated controlled environments — Part 3: Test methods in an effort to improve the document and thus enhance credibility and resulting quality.
Changes in Classification Method
The present ISO 14644-1 document depends on an ad-hoc approach to ascertain the location and number of sampling areas for classifying cleanrooms. This method, however, is not built on statistical sampling technique and also does not consider any risk-based evaluation. At present, the working group is recommending a randomized sampling location selection technique, in tandem with a risk-assessed, fixed-location sampling technique.
Founded on the hypergeometric statistical model, the randomized sampling technique enhances confidence that the selected sample provides 95% assurance that the results represent 90% of all areas in the cleanroom. Examining the additional risk, the fixed sampling location technique enables cleanroom users to choose the areas where their product is believed to be most at risk. When the cleanroom is classified first, these locations will be sampled, and whenever the room is re-qualified the locations will be sampled again.
When combined together, these two methods provide improved confidence that the cleanroom is operating as expected and ideal for providing the right quality-controlled environment for life science applications. These methods are better than the method specified in the existing ISO 14644-1 document.
Improving Confidence in Quality
In addition to cleanroom monitoring and classification applications in the life sciences and pharmaceutical industries, there are certain particle counters that can be utilized in indoor air quality and other less critical industrial applications.
Figure 1. Particle counters can be designed for different applications. Image credt: Beckman Coulter
Although a number of air particle counters available in the market are designed for use in all three application areas, some give incorrect results in low particle concentration environments such as life-critical cleanroom applications, while others are unable to handle high particle population environments found in the industrial applications. Therefore, particle counters developed for the less critical industrial applications may not meet the rigorous requirements specified in ISO 21501-4 and ISO 14644-3.
In an effort to enhance confidence in the quality of cleanroom environments, the amended ISO 14644-1 document will comprise the performance criteria of particle counter specified in ISO 14644-3 and give a normative reference to ISO 21501-4, Determination of particle size distribution — Single particle light interaction methods — Part 4: Light scattering airborne particle counter for clean spaces, to guide the reader through a consistent test method for the calibration of particle counters. The ISO 21501-4 document was initially published in 2007.
Additionally, manufacturers of particle counters and national standards agencies have publicized the normative reference to ISO 21501-4 in the amended document ever since the initial draft of the revised ISO 14644-1 document was published in December 2010. Therefore, the working group believed that, by the estimated publication date of the amended ISO 14644-1 in 2013, the industries using the ISO 14644-1 document would have sufficient notification of the need to conform to the ISO 21501-4 calibration method and to also upgrade any particle counters that are non-compliant.
Prior to the publication of ISO 21501-4 in 2007, no calibration standards were available for airborne particle counters which resulted in fluctuating levels of performance from particle counting systems. Since 2007, many particle counter manufacturers across the globe have modified the designs of their particle counters meant for critical life science ISO 14644 applications, so that these instruments can fulfill the more complex expectations of the latest calibration standard. Now, cleanroom users can have improved confidence that their cleanrooms are providing the required level of quality-controlled environment for their specific processes.
Content Imported from ISO 14644-3
The performance requirements of particle counter have been laid down in the ISO 14644-3 document since its first publication in 2005 and corresponds with the ISO 21501-4.
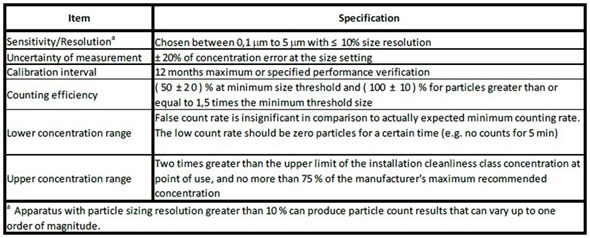
Figure 2. Specifications for the light-scattering discrete-particle counter from ISO 14644-3. Image credt: Beckman Coulter
In an effort to enhance clarity, the International Standards Organization Technical Committee ISO TC 209 determined that particle counter performance methods and criteria used to attain classification should be integrated into each ISO 14644 standard. Therefore, the performance criteria of particle counter specified in Table C.1 of ISO 14644-3 will appear in the amended ISO 14644-1 document.
Publication timeline
The initial publication of the Draft International Standard (DIS) DIS ISO 14644-1 in December 2010 triggered a heated debate. After assessing the questions and comments from the international community with regard to the DIS, the expert working group planned to publish an amended draft DIS document in 2013. In the interim, ISO Technical Committee TC209 recommends that users who want to leverage the improvements in the 2010 DIS ISO 14644-1 document can use it, as long as they know that the final published version will be modified.
Conclusion
The improved sampling method in the revised ISO 14644-1 document along with the enhanced guidance for particle counter calibration and performance will provide improved confidence to cleanroom users that their cleanroom is delivering the preferred level of quality-controlled environment for critical life science applications.
 About Beckman Coulter
About Beckman Coulter
Beckman Coulter develops, manufactures and markets products that simplify, automate and innovate complex biomedical tests. More than a quarter of a million Beckman Coulter instruments operate in laboratories around the world, supplying critical information for improving patient health and reducing the cost of care.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.