A recent report indicated that in 2016, around 79% of 483 warning letters from the FDA to the pharmaceutical industry highlighted weaknesses in data integrity.1 The FDA outlines its expectations for quality critical instrumentation in the GMP environment within the 21CFR part 11 ruling.2
This article explores the way in which quality-critical on-line Total Organic Carbon (TOC) alongside conductivity instrumentation for WFI (water for injection) quality control may be configured to assist companies in complying with the FDA’s data integrity requirements. This includes the use of Microsoft Active Directory and data export via PDF.
Background
TOC and conductivity are two of the four core quality factors specified for WFI and PW (purified water) in the United States Pharmacopoeia.3 It is possible to validate on-line analyzers like the ANATEL PAT700 from Beckman Coulter to achieve full compliance for both of these quality attributes, in line with the FDA’s pharmacopoeial requirements.
The FDA Process Analytical Technologies (PAT) initiative4 has urged the pharmaceutical industry to invest in process control instrumentation designed to ensure in-process quality control, instead of relying on final product quality testing. Right-first-time is considered the ideal, because it is not possible to apply final product quality testing to 100% of batches. This is because the tests are generally destructive.
With this is mind, a large number of pharmaceutical manufacturers are connecting on-line TOC analyzers to factory control systems, allowing potential TOC or conductivity excursions which are detected to be used to halt production. This eliminates the risk of contaminated water being mixed with important active pharmaceutical ingredients.
Following revisions to the European Pharmacopoeia (EP) chapter, including WFI now enabling the production of WFI from ultra-filtration and double pass reverse osmosis (RO),5 TOC and conductivity monitoring of pharmaceutical water systems has become increasingly important due to the potential risk of contamination break-through in the RO system, especially when compared to the secure barrier provided by a water still.
FDA ALCOA guidance
The FDA uses the acronym ALCOA in its 2003 guidance on the implementation of their 21CFR part 11 data integrity rule. This section focuses on defining good data integrity practice, citing this as the creation of records which are attributable to the technician carrying out the testing, legible, created contemporaneously created, original and accurate.

The FDA 21CFR part 11 ALCOA definition of complete, consistent and accurate data. Image Credit: Beckman Coulter Life Sciences
Attributable
Attributable may be understood to imply that records would ideally include an electronic ‘signature’ which links them to the user or instrument making the measurement. This could also mean that they should include a reference to the water system being tested, as well as the date and time the measurement was taken.
An electronic signature should be created for every user signed on to the system. Control over the format of electronic signatures can be site specific and this is commonly controlled by the company’s IT department via Microsoft Active Directory controls.
Preferably, the on-line instrumentation would be configured to follow Active Directory controls, as this would enable correct electronic signature formats to be maintained according to site-specific rules.
Legible records
The record must be legible, which suggests that hand-written records are not appropriate. The FDA goes on to indicate that electronic records must be stored in a format that is open and compatible with a wide range of computer platforms and applications. The FDA recommends common formats such as XML, SGML or PDF.
Contemporaneous
The word contemporaneously suggests that electronic records should be created as soon as the sample is measured. Manual transcription of paper records is not considered to be good practice, nor is the collation of paper records for transcription into an electronic format at a later time or date.
There is an element of risk with every transcription of test results from one format to another, and even scanning numerous paper records into electronic formats has the potential for missed scans or duplication.
The FDA advises that the electronic record must be an original record, created as soon as the test is completed. In this instance, manually transcribed records carry the greatest risk and the greatest opportunity for human error.
Accurate records
Lastly, the A in ALCOA outlines the need for electronic records to be accurate. This suggests that the process for capturing electronic records must be robust, avoiding manual calculations and manual data entry where the clear risks of human error are present.
Attributable records
The ‘A’ in ALCOA stands for ‘attributable’. Electronic records from on-line instruments must include information that links the data to the instrument used to make the measurement, as well as the time and date of that measurement.
On-line instruments that are able to analyze grab samples (like the ANATEL PAT700) should allocate the grab sample analysis data to the user who carried out the test. This is commonly done via electronic signatures.
User accounts should function at multiple levels, with day-to-day users not required to log on to view up-to-date on-line TOC results. However, users who wish to alter settings, undertake calibrations or complete system suitability tests should be required to log on with appropriate credentials.
Once users are logged on to the system, they should be automatically logged off after a configurable period of inactivity.
Additionally, users should be required to change their passwords within a predetermined and regular timeframe, with the use of previous passwords not permitted.
Controls enforced by the site IT team and appropriately defined in the Microsoft Active Directory control should be mirrored in the on-line TOC analyzer.
Data repositories
The FDA’s guidance places great emphasis on the fact that the 21CFR part 11 ruling applies only to the data historian where electronic records are stored. The primary risk with on-line instruments that include their own, built in local data historian is that they may attract all the requirements outlined in the 21CFR part 11 ruling.
Analyzers like the PAT700 avoid this issue by allowing the local data historian to be disabled, thus ensuring that it does not attract the full 21CFR part 11 requirement as a data archive for electronic records.
Data from on-line TOC analyzers is commonly held in validated Distributed Control Systems (DCS) and Supervisory Control And Data Acquisition (SCADA) systems. This has historically made improvements to process control challenging, adding a substantial amount of change control.
However, contemporary approaches store quality critical data records in a separate secure archive, meaning that SCADA and DCS systems can be dedicated to process control only, thus making them more agile.
The PAT700 is able to support this. The instrument can be configured to automatically send PDF electronic records via secure FTP over Ethernet, meeting the ALCOA requirements for all electronic records to be legible, contemporaneous, original and attributable.
The archiving of reports by sending these to a remote, secure data archive over Ethernet via secure FTP may be done automatically, with PDF files automatically exported and archived at predetermined intervals.
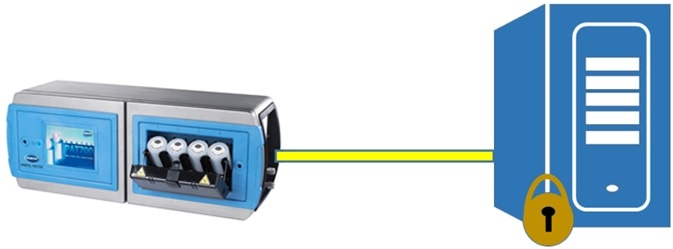
Beckman Coulter ANATEL PAT700 exports the PDF file straight to data archive via Ethernet. Image Credit: Beckman Coulter Life Sciences
Manual SOPs versus electronic SOPs
The ANATEL PAT700 satisfies ALCOA’s ‘A’ for accuracy on several levels. Manual processes are removed, with all necessary SOPs automated and pre-programmed into the analyzer itself, using an electronic SOP format.
No manual calculations are required when performing calibrations or system suitability testing. No manual data entry is required either, as certified standards values, expiry dates and lot numbers are automatically entered into the analyzer via RFID tags on the standards bottles.
Manual calculations
Human error resulting from manual calculations for pass/fail reports can adversely affect the ‘A’ for accuracy and ‘C’ for contemporaneous within the ALCOA requirements. Best practice dictates that calculations for pass/fail be built into the analyzer itself, enabling automatic pass/fail reports to be calculated and generated within the instrument.
The PAT700 includes this pass/fail criteria, automatically generating pass/fail reports in PDF format, and satisfying ALCOA’s requirement for accurate, contemporaneously generated records.
While a great deal of attention is given to the final electronic record’s security, many opportunities exist whereby incorrect records stem from the use of manual processes, such as manual calculations and manual data entry.
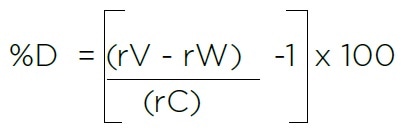
Manual calculations in particular may present opportunities for human error where:
- rV = Average TOC response for three measurements of the Sucrose Validation Standard
- rW = Average TOC response for three measurements of the Sucrose Validation Standard
- rC = Certified TOC value from the Certificate of Analysis for the Validation Standard
Calibrations
Calibrations undertaken with manual SOPs and manual calculations also present a considerable opportunity for human error. The Beckman Coulter ANATEL PAT700 includes all necessary SOPs in electronic format, with calibration standards automatically importing their certified value, lot number and expiry date into the PAT700 via RFID. All of this key data is included in the calibration report in PDF format.

Closed view of the ANATEL PAT700 Total Organic Carbon Analyzer. Image Credit: Beckman Coulter Life Sciences
Retraining versus robust processes
A typical response to data errors is to insist on retraining the team. However, both the industry and the FDA are starting to realize that this does not solve the problem - it simply treats the symptoms until human error begins to appear again. Instead, the correct approach involves a reduction in the amount of manual steps in the SOP, allowing human errors to be reduced while resulting in a whole process that is more robust.
Image Credit: Beckman Coulter Life Sciences
Conclusion
Vital on-line water quality instrumentation is becoming increasingly important as the rules on WFI generation are loosened within the European Pharmacopoeia. Manual calculations and paper-based SOPs provide too much potential for human error, while retraining can be understood as treating the symptoms without curing the problem.
The technology to make these instruments more robust is available however, and by automating SOPs while eliminating manual calibrations via instrumentation optimized for pharmaceutical quality control - such as the Beckman Coulter ANATEL PAT700 TOC and conductivity analyzer – this can be accomplished.
By focusing on cost control and optimization, users are better placed to consider making their on-line quality control instrumentation more robust in order to prevent any loss of valuable active pharmaceutical ingredient product. This is particularly important for those in the biopharmaceutical industry.
References
- Pharmaceutical Online, An Analysis Of FDA FY2016 Drug GMP Warning Letters By Barbara Unger, Unger Consulting Inc. https://www.pharmaceuticalonline.com/doc/an-analysis-of-fda-fy-drug-gmp-warning-letters-0001
- U.S. Department of Health and Human Services Food and Drug Administration Guidance for Industry, Part 11, Electronic Records; Electronic Signatures — Scope and Application August 2003 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA
- U.S. Department of Health and Human Services Food and Drug Administration United States Pharmacopoeia U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA
- U.S. Department of Health and Human Services Food and Drug Administration Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance September 2004 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/liposome-drug-products-chemistry-manufacturing-and-controls-human-pharmacokinetics-and
- Council of Europe European Directorate for the Quality of Medicines & Healthcare European Pharmacopoeia (Ph. Eur) 9th Edition. EDQM Council of Europe, 7 allée Kastner, CS 30026, F-67081 Strasbourg, France https://www.edqm.eu/en/news/shutdown-european-pharmacopoeia-9th-edition
About Beckman Coulter Life Sciences
Beckman Coulter Life Sciences is dedicated to empowering discovery and scientific breakthroughs. The company’s global leadership and world-class service and support delivers sophisticated instrument systems, reagents and services to life science researchers in academic and commercial laboratories, enabling new discoveries in biology-based research and development.
A leader in centrifugation and flow cytometry, Beckman Coulter has long been an innovator in particle characterization and laboratory automation, and its products are used at the forefront of important areas of investigation, including genomics and proteomics.
Primary activity / Product lines
- Flow Cytometry
- Centrifugation
- Particle Counting and Characterization
- Liquid Handling and Robotics
- Nucleic Acid Sample Preparation
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.