With an easy-to-use instrument to encapsulate single cells in picolitre-volume water droplets (picodroplets) at high throughput rates of thousands of droplets per second, picodroplet microfluidics is quickly emerging as the technology of choice for single-cell analysis.
This highly effective and delicate method has important benefits in terms of cost and effectiveness, such as:
- High-throughput workflow: They enable the isolation and analysis of tens of thousands to millions of cells in a single experiment
- Precisely defined microenvironment: For individualized engineering, screening, and characterization, they compartmentalize individual cells in a regulated environment
- Highly concentrated volumes: They reduce diffusion while increasing detection sensitivity with higher concentrations of essential ingredients held in picodroplets
- Low operating costs: They cut reagent costs by miniaturizing crucial stages to a picolitre volume
- Integration and automation: They provide a low-effort, low-risk way to establish optimized workflows and cut back on labor-intensive operations
Picodroplets, which are used in this method, offer a culturing environment that is hospitable to cells, which is another benefit. In these picodroplets, cells can be grown for a few hours up to several days, depending on the test and application of interest.
Numerous applications, including the development of antibodies, cell line cloning, and cell engineering, are now possible due to the capacity to cultivate the cells for varying amounts of time.
This article provides an overview of the potential applications of picodroplets in cell engineering; it will first evaluate the compatibility of reagents for chemical-based transfection with picodroplets. It will then introduce editing steps of increasing complexity to address the various viral and non-viral delivery systems utilized in the research community.
These steps progress from conventional plasmid-based transfection to viral-based transfection and conclude with CRISPR-Cas9 delivery into mammalian cells using automated instrumentation.
Compatibility of cells and reagents with picodroplets
A variety of commonly used transfection reagents, such as liposome-cationic lipids, non-liposomal lipids, activated dendrimers, and lipopolyplexes (Figure 1A), were chosen for compatibility testing with picodroplets technology prior to the genome editing research.
In this test, 400 pL picodroplets were produced using syringe pumps after the transfection reagents were combined with the encapsulating medium in a ratio of 1:100 to imitate the conventional transfection procedures.
After 2 and 24 hours of incubation at 37 °C, the stability of the picodroplet was then evaluated under a microscope (Figure 1B). According to the findings, various transfection reagents have no effect on picodroplet production or stability in any of the studies.
A)
| Group of reagents |
Specific reagent |
Supplier |
| Liposomes-cationic lipids transfection |
Lipofectamine & Lipofectamine 3000 |
Thermo Fisher Scientific |
| Non-liposomal lipids |
Fugene |
Promega |
| Activated dendrimers |
PolyFect |
Qiagen |
| Lipopolyplexes |
TransIT |
Mirus |
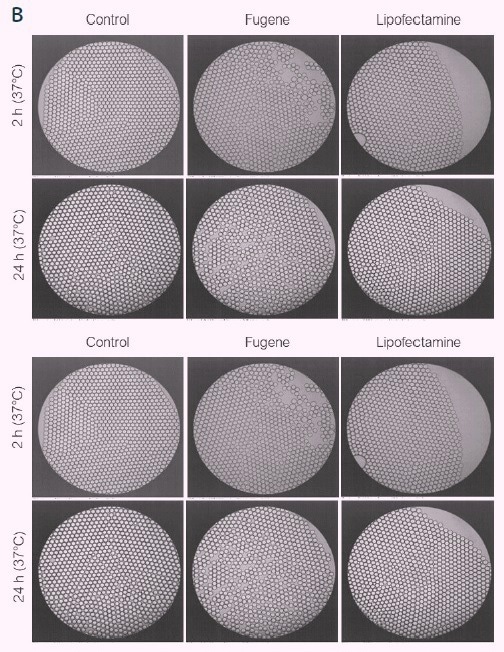
Figure 1. Compatibility of picodroplets with different transfection reagents. Image Credit: Fluidic Sciences and Sphere Bio
Cell engineering in picodroplets experiments
Employing various viral and non-viral delivery techniques, cell engineering in picodroplets was accomplished effectively. These include the automated delivery of CRISPR-CaS9 into mammalian cells, viral-based transfection, and plasmid-based transfection.

Cyto-Mine® - Fluidic Sciences and Sphere Bio’ automated and fully integrated single cell analysis system. Image Credit: Fluidic Sciences and Sphere Bio
Plasmid-based transfection in picodroplets
HCT116 cells (2.5 × 105 cells) and a transfection cocktail of 0.05 µg DNA (GFP)/0.1 µl Lipofectamine 3000/150 µl medium were co-encapsulated in 300 pL picodroplets for a plasmid-based transfection investigation (Figure 2A). Pre-seeded cells were transfected with the same transfection mixture in accordance with the manufacturer’s instructions as a control.
Cells and transfection agents were contained in picodroplets, which were incubated at 37 °C for 2 hours. By chemically causing the coalescence of the picodroplets, Pico-Break™ was used to extract the cells from the picodroplets. The retrieved cells were centrifuged and cleaned before being cultivated once again in a new culture medium.
After 24 hours of cell culture, fluorescent images were captured to determine the success rate of cell transfection. Quantitative assessments of GFP protein expression levels were made for both the control and picodroplet samples after 48 hours of culture (Figure 2B).
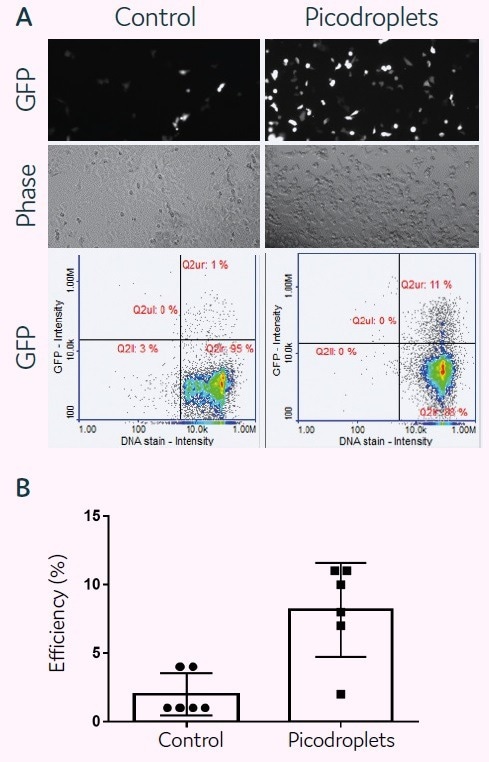
Figure 2. plasmid-based transfection of HCT116 mammalian cells in picodroplets. Image Credit: Fluidic Sciences and Sphere Bio
NucleoCounter® NC3000™ Advanced Image Cytometer images and performs quantitative evaluations of GFP expression in transfected cells. The quantitative analysis of six distinct trials shows that transfection performed well in picodroplets and could be even more effective than in bulk transfection.
Viral transduction in picodroplets
Jurkat cells (1 × 106 cells) and EGFP-Lenti Genecopoeia virions (MOI = 2) were co-encapsulated in 400 pL picodroplets. Picodroplets were cultivated for 48 hours at 37 °C and then studied under a fluorescent microscope. The efficiency of transduction was assessed by measuring GFP expression using the Single Cell Assay and Isolation System from Fluidic Sciences and Sphere Bio.
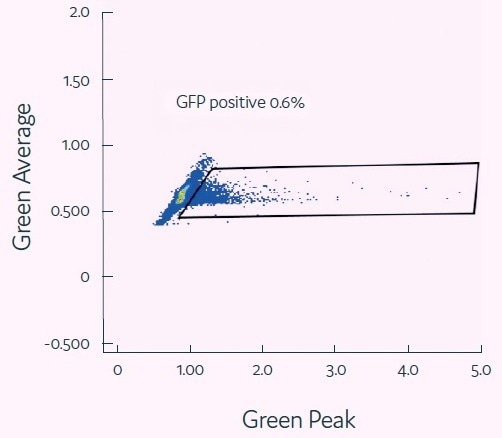
Figure 3. Viral transduction of mammalian cells. Image Credit: Fluidic Sciences and Sphere Bio
mRNA transfection in picodroplets
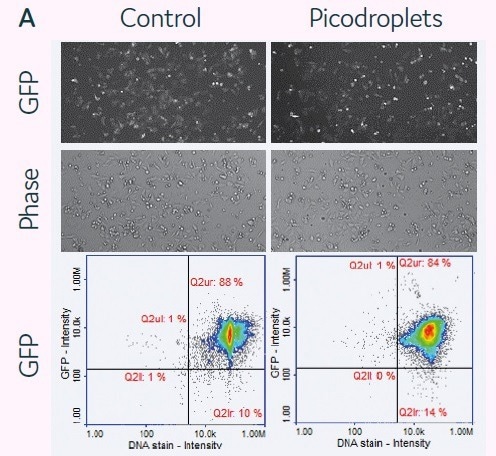
The viability of mRNA delivery into mammalian cells in picodroplets was examined next. For this purpose, 2.5 × 105 HCT116 cells were combined with a transfection cocktail (200 ng of GFP mRNA + 0.4 µL/150 µl sample of DharmaFect Duo) (Figure 4A). In accordance with the manufacturer’s protocols (control), cells were reverse-transfected in parallel using the same transfection cocktail.
Then, cells in picodroplets were treated at 37 °C for 4 hours. Using Pico-Break™, cells were extracted from the picodroplets and centrifuged before being cleaned and cultivated in a new culture medium.
Following a 24-hour culture period, the expression of the GFP protein was measured using the fluorescent microscopy method or the NucleoCounter® 3000 quantification methodology. It was shown that the expression levels between the conventional and picodroplet-based approaches were similar (Figure 4A).
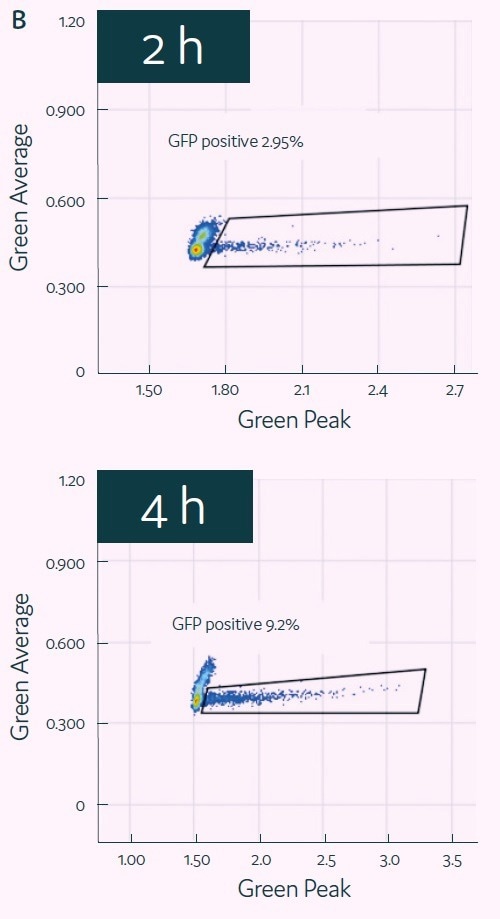
HCT116 cells and transfection mix were encapsulated into picodroplets using Cyto-Mine® to test its capacity for detecting and isolating GFP-transfected cells. GFP expression of the encapsulated cells was then evaluated after two to four hours of incubation. (See Figure 4B.)
GFP signal peaked after four hours, at which point positive picodroplets were selected and placed onto 96-well microtiter plates. After two weeks of outgrowth, the percentage of wells that generated colonies of cells was assessed (Figure 4C), demonstrating the successful completion of the process that begins with picodroplet-based mRNA transfection and ends with the effective outgrowth of sorted single cells.
C)
| Plate # |
Recovery (%) |
| 1 |
84.78 |
| 2 |
95.40 |
| 3 |
71.59 |
| 4 |
82.28 |
| 5 |
79.75 |
| average |
82.76 |
| stdev |
8.6 |
Figure 4. mRNA transfection of mammalian cells in picodroplets. Image Credit: Fluidic Sciences and Sphere Bio
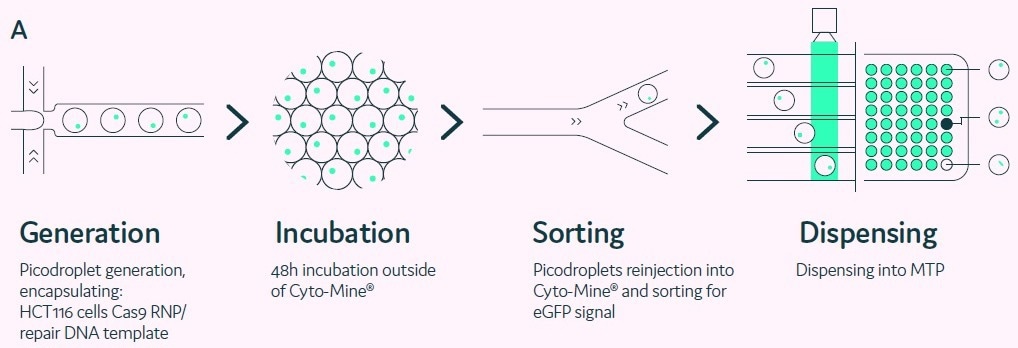
Figure 5. Genome-editing of mammalian cells in picodroplets. Image Credit: Fluidic Sciences and Sphere Bio

 Download the full paper
Download the full paper
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.