Multiplexing is a powerful tool in biological research and development, enabling the simultaneous analysis of multiple parameters or targets within a single experiment.
This is a summary - Download the full application note here
This approach offers several key advantages, including higher throughput, improved assay accuracy, reduced sample requirements, greater cost efficiency, and deeper insights into complex biological systems.1,2
As this technology advances, researchers are increasingly utilizing combined assays to gain deeper insights into complex biological processes - insights that are fueling innovation across the biotechnology and healthcare sectors.3,4,5,6,7,8
Despite its advantages, multiplexing also presents challenges, such as the need for specialized equipment and assays, advanced software and data analysis tools, and the potential for cross-reactivity, which can lead to false positives.1,2

Image Credit: Fluidic Sciences and Sphere Bio
Fluidic Sciences and Sphere Bio’ proprietary picodroplet technology has been specifically designed to enable automated, high-throughput screening and selection of single cells based on secreted molecules or surface markers.9
The first-generation Cyto-Mine® uses a powerful fluorescent assay powered by a single excitation laser and two detection channels to facilitate high-throughput single-cell analysis.
Increasing the number of excitation lasers and detection channels would enhance fluorescence multiplexing, improving assay specificity and enabling more precise target selection and high-throughput screening.
Fluidic Sciences and Sphere Bio has introduced the Cyto-Mine® Chroma to address this key research need. The company’s next-generation Cyto-Mine® instrument features multiple lasers and detectors, enabling automated single-cell analysis and isolation with cutting-edge multiplexing capabilities.
This article showcases the potential of spectral multiplexing within droplets, particularly focussing on its usefulness in antibody discovery (AbD) and cell line development (CLD) applications.
To assess the performance and capabilities of the Cyto-Mine® Chroma, multiplexed biological assays were designed to enable the simultaneous analysis of multi-color parameters. The results demonstrate that Cyto-Mine® Chroma effectively identifies both secreted and cellular markers in fluorescence-based multiplexed assays.
Cells were identified and isolated based on productivity (IgG secretion) and antigen specificity (antibody-antigen binding) while maintaining high accuracy.
In alignment with AbD workflows, the findings confirm that Cyto-Mine® Chroma enables highly precise detection, analysis, and isolation of rare, antibody-secreting single cells from a heterogeneous starting population.
Aims and objectives
- Demonstrate the detection and isolation of cell subpopulations based on a fluorescent cell label and IgG secretion, measured using Förster Resonance Energy Transfer (FRET) signals.
- Demonstrate the isolation of rare antibody-secreting cells through the binding of secreted antibodies to surface-expressed antigens in a multiplexed assay.
Summary of results
Accurate detection, sorting, and dispensing are critical for isolating rare antibody-secreting cells from a mixed cell population. These cells can be isolated based on the presence of secreted IgG or the specific binding of secreted IgG to its antigen.
This study evaluated the Cyto-Mine® Chroma’s ability to successfully isolate these cells. The findings confirmed that single-cell isolation was achievable, whether droplets were sorted using single or sequential gating. Both secretion analysis and cell staining were employed to effectively gate the population of interest.
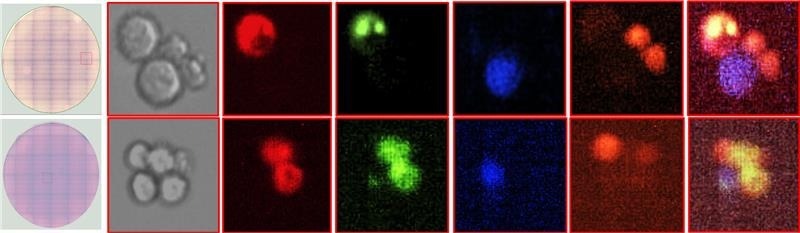
Identifying and isolating antigen-specific antibody-secreting cells in a mixed population containing unrelated secreting cells, thanks to Cyto-Mine® Chroma. Image Credit: Fluidic Sciences and Sphere Bio
The study also demonstrated the accurate detection of antibody binding to surface-expressed antigens, with detection specificity highlighted using a recombinant antibody. Rare cells of interest were successfully isolated through multiplexed antigen-binding detection, with additional labeling of antibody-secreting cells (ASC) and target cells.
Furthermore, even in cultures containing two different ASC populations, the system accurately isolated the desired cells based on the binding of the secreted antibody to its target cell.
Conclusions
The results presented here demonstrate the Cyto-Mine® Chroma system’s robust multiplexing capabilities, along with its ability to accurately detect and isolate cells of interest using picodroplets.
This advanced multi-laser instrument enables sequential gating, precise sorting, and dispensing based on fluorescent signals from secreted antibodies or encapsulated cells.
The study confirmed that droplets containing rare cells of interest could be accurately isolated based on antibody-antigen binding, even in complex secretion assays where the relevant cells represent only a small fraction of the total population.
These capabilities can be summarized into three key areas:
Detection accuracy
The platform reliably detected both localized fluorescence (antibody-antigen binding) and dispersed fluorescence (IgG secretion, FRET signal). When combined with fluorescently stained cells, it accurately distinguished between different cell types based on their specific staining.
Sorting accuracy of gated populations
The system demonstrated high sorting accuracy and the ability to enrich target populations following either single or sequential gating.
Multiplexing capability
The Cyto-Mine® Chroma exhibited excellent multiplexing performance, successfully assessing and isolating rare secreting cells by simultaneously detecting four different cellular markers.
This is a summary - Download the full application note here
References and further reading
- Mills, P.J. and Peterson, C.T. (2016). Multiplexing and Beyond in Biobehavioral Research. Psychosomatic Medicine, (online) 78(6), pp.642–645. https://doi.org/10.1097/psy.0000000000000329.
- Chen, J. and Schwarz, E. (2016). Opportunities and Challenges of Multiplex Assays: A Machine Learning Perspective. Methods in molecular biology, (online) pp.115–122. https://doi.org/10.1007/978-1-4939-6730-8_7.
- Chen, J., Guest, P.C. and Schwarz, E. (2017). The Utility of Multiplex Assays for Identification of Proteomic Signatures in Psychiatry. Advances in experimental medicine and biology, (online) 974, pp.131–138. https://doi.org/10.1007/978-3-319-52479-5_8.
- Edwards, B.S., et al. (2007). High‐Throughput Cytotoxicity Screening by Propidium Iodide Staining. Current Protocols in Cytometry, 41(1). https://doi.org/10.1002/0471142956.cy0924s41.
- Krutzik, P.O. and Nolan, G.P. (2006). Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nature Methods, 3(5), pp.361–368. https://doi.org/10.1038/nmeth872.
- Bray, R.A., et al. (2020). Development and Validation of a Multiplex, Bead-based Assay to Detect Antibodies Directed Against SARS-CoV-2 Proteins. Transplantation, 105(1), pp.79–89. https://doi.org/10.1097/tp.0000000000003524.
- Edwards, B.S., et al. (2007). High-throughput flow cytometry for drug discovery. Expert opinion on drug discovery, 2(5), pp.685–696. https://doi.org/10.1517/17460441.2.5.685.
- Brouzes, E., et al. (2009). Droplet microfluidic technology for single-cell high-throughput screening. Proceedings of the National Academy of Sciences, 106(34), pp.14195–14200. https://doi.org/10.1073/pnas.0903542106.
- Dimitris Josephides, Davoli, S., et al. (2020). Cyto-Mine: An Integrated, Picodroplet System for High-Throughput Single-Cell Analysis, Sorting, Dispensing, and Monoclonality Assurance. SLAS TECHNOLOGY, 25(2), pp.177–189. https://doi.org/10.1177/2472630319892571.
Acknowledgments
Produced from materials originally authored by Jitender Bisht, Elena Shvets, Maryam Ahmadi, and Richard Hammond from Fluidic Sciences and Sphere Bio.
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.