Scientists at FUJIFILM Diosynth Biotechnologies have transformed their cell line development processes by incorporating Cyto-Mine®, the next-generation single-cell analysis system developed by Fluidic Sciences and Sphere Bio.
Compared to past workflows, the new procedures are simple and productive. Projects are now able to transition swiftly from the initial transfection phase through to the development of highly productive cell lines within around ten weeks.
Cell line development: Critical objectives and challenges
Specialist contract development and manufacturing organizations (CDMOs) offer technical expertise and capacity in cell line and process development to optimize the production of biological medicines for biotherapeutic manufacturers.
In this commercially competitive industry, CDMOs are under mounting pressure to provide robust and prolific stable cell lines in shorter durations to expedite the transfer of medicinal compounds to clinical research. This must be accomplished without compromising the quality of biological products.
Innovative antibody fragments and bispecific antibodies are challenging to manufacture in stable cell line expression systems at adequate titers for commercial drug research programs and large-scale production compared to conventional monoclonal antibody molecules.
Technologies that offer enhanced predictive screening capabilities and higher throughputs than those conventionally used in cell line development laboratories have been sought after to meet the growing urgency and demand for unique biotherapeutic molecules.
Traditional manual screening approaches, such as colony picking and restricting dilution cloning, are laborious, time-consuming, and limited in terms of the number of cells that can be treated or evaluated in a given time frame.
Industry regulators, such as the Food and Drug Administration (FDA), require evidence of monoclonality regarding cell lines used in the production of biological medicines.
To prevent expensive delays in market authorization and clinical trial programs, manufacturers must demonstrate that each cell line is produced from a single parent cell.
Conventional methods do not allow visualization of individual cells upon initial seeding, making the demonstration of monoclonality difficult or impossible in certain cases.

Image Credit: Fluidic Sciences and Sphere Bio
Cyto-Mine® in practice: Improving efficiency and ensuring quality
Cyto-Mine® is a platform for the single-cell investigation that permits the encapsulation of individual cells inside picolitre-sized aqueous droplets in a biocompatible carrier oil (pico-droplets).
This technique has been used by FUJIFILM Diosynth Biotechnologies to produce a single-step cloning procedure that eliminates bottlenecks in the cell growth process and enhances accuracy and efficiency.
Each pico-droplet offers a regulated, delimited, and modifiable environment that preserves cell viability (Figure 1). Pico-droplets entrap secreted proteins, making them readily accessible for analysis and enabling rapid screening of single cells.
Multiple processes and tests throughout the cell line production pathway are downsized and integrated into Cyto-Mine®, enabling the completion of activities in a single day that would have otherwise taken weeks.
Utilizing this method, around 200,000 individual cells can be examined in a couple of hours, compared to approximately 10,000 cells using manual analytic approaches.
Screening is performed early on so that only cells expressing the desired protein and the best-performing cells within the pool are advanced in the development process, therefore enhancing the quality and quantity of molecular products.
Workflows have been drastically reduced from around 25 weeks to about ten weeks (Figure 2). Moreover, utilizing Cyto-Mine®, single cells can be viewed and correct monoclonality data can be supplied to assist regulatory applications.
Cyto-Mine® is built with user-friendly, integrated software that needs minimum specialized training to comprehend and use efficiently.
Cyto-Mine® has increased the overall effectiveness and productivity of the cell line and method development team at FUJIFILM DiosynthBiotechnologies. Scientists spend less time doing tedious manual methods and can accomplish more work within a given time frame.
This enables them to devote their specialized knowledge and talents to other crucial parts of their role. Scientists at FUJIFILM Diosynth Biotechnologies are already investigating further uses for this technological platform, inspired by the potential of Cyto-Mine® to improve or refine other facets of their study.
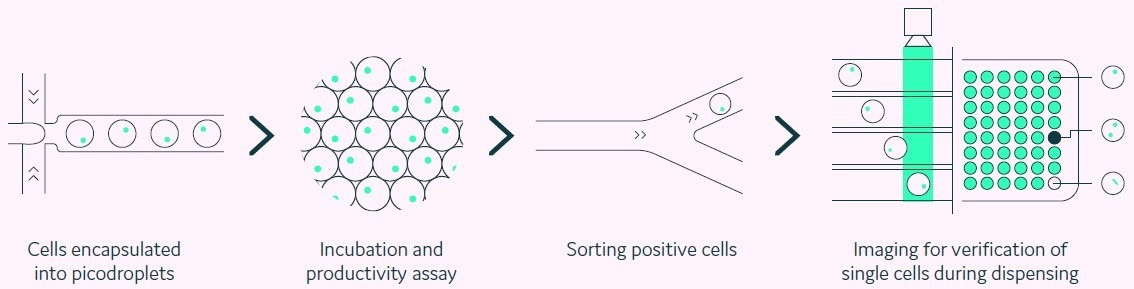
Figure 1. Integration of productivity assay screening, sorting, isolation and verification using a fully automated picodroplet microfluidic process. Image Credit: Fluidic Sciences and Sphere Bio

Figure 2. Example timeline highlighting the streamlining of workflows using mammalian cell line engineering technologies in combination with single-cell picodroplet innovations. Image Credit: Fluidic Sciences and Sphere Bio
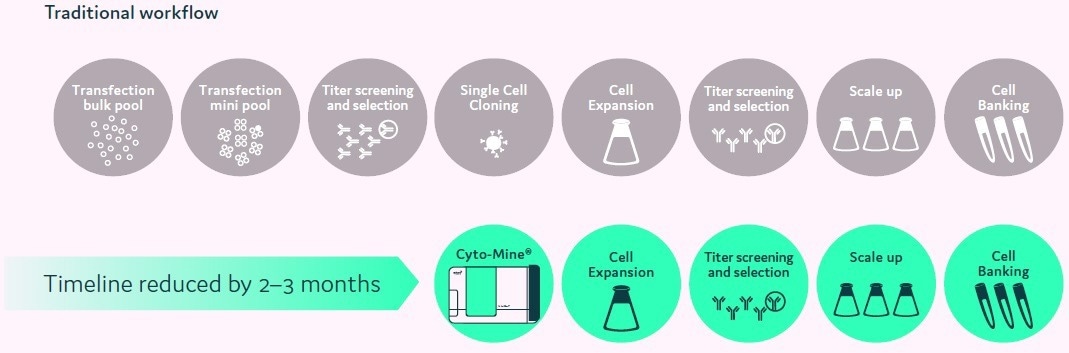
Figure 3. Accelerated cell line development workflow using Cyto-Mine®. Image Credit: Fluidic Sciences and Sphere Bio
The benefits of picodroplet technology
- Streamlined and simplified processes
- Miniaturization and integration of multiple processes/pathways
- Accurately screening large cellular populations (200,000 individual cells)
- Shortened timelines/workflows
- Assured monoclonality
- Increased efficiency
- Automated processing and greater freedom from manual tasks

 Download the full paper
Download the full paper
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.