In recent years, cell therapy has gained significant attention as a method for utilizing the immune system in the fight against tumors. This is achieved through the reprogramming of T-cells collected from patients to create personalized medicines.
T-cell therapy leverages the body's innate ability to identify and eliminate tumor cells, which can become lost or overpowered as cancer progresses. Extracting and modifying a patient's T-cells makes it possible to restore the ability to attack cancer cells.
For the effective and efficient development of these therapies, rapid and accurate assays are necessary to assess the potency of the modified T-cells against the target tumor.
This article introduces a novel picodroplet-based method for validating the functionality of CAR-T cells. This user-friendly and robust approach combines the advantages of Fluidic Sciences and Sphere Bio' high-throughput picodroplet technology with a granzyme B assay to study cell-mediated cytotoxicity (as shown in Figure 1).
The engineered T-cells and target cells are co-encapsulated within picoliter-volume droplets in an oil emulsion, along with granzyme B substrate.
The release of Granzyme B by the T-cell, which serves as an indicator of the cell's ability to recognize and eliminate the cancer cell, can be monitored to assess its potential anti-cancer activity.
This method allows for rapid cell product efficacy assessments, thereby streamlining the quality control release process of the cell product.

Figure 1. Workflow depicting high throughput CAR-T cell function verification in microfluidic picodroplets. Image Credit: Fluidic Sciences and Sphere Bio
About picodroplet technology
The development of picodroplet-based technology has positioned it as a highly appealing microfluidic technique for single-cell functional analysis. This method offers several benefits compared to conventional tools.
Picodroplets offer a distinct microenvironment for high-throughput analysis of cell secretion and cell-cell interaction studies at the single-cell level.
The miniaturization of the picoliter volumes results in faster mixing and reduced sample dilution, thereby increasing detection sensitivity and minimizing sample requirements and reaction times.
About Granzyme B detection in picodroplets
The detection of Granzyme B release in picodroplets is achieved through the use of the commercially available SensoLyte® Granzyme B Activity Assay Kit, developed by Anaspec in Fremont, CA. This assay has been adapted for use in picodroplets.
The assay operates on the principle of cleavage of a Granzyme B substrate peptide labeled with a 5-FAM fluorophore and a QXL®-520 fluorophore quencher. In the absence of cleavage, no fluorescence is emitted due to the quenching effect of the adjacent QXL®-520 molecule (as illustrated in Figure 2A).
In the presence of Granzyme B, the substrate peptide is cleaved, releasing the quenching molecule and resulting in a fluorescent signal (as shown in Figures 2A and B).
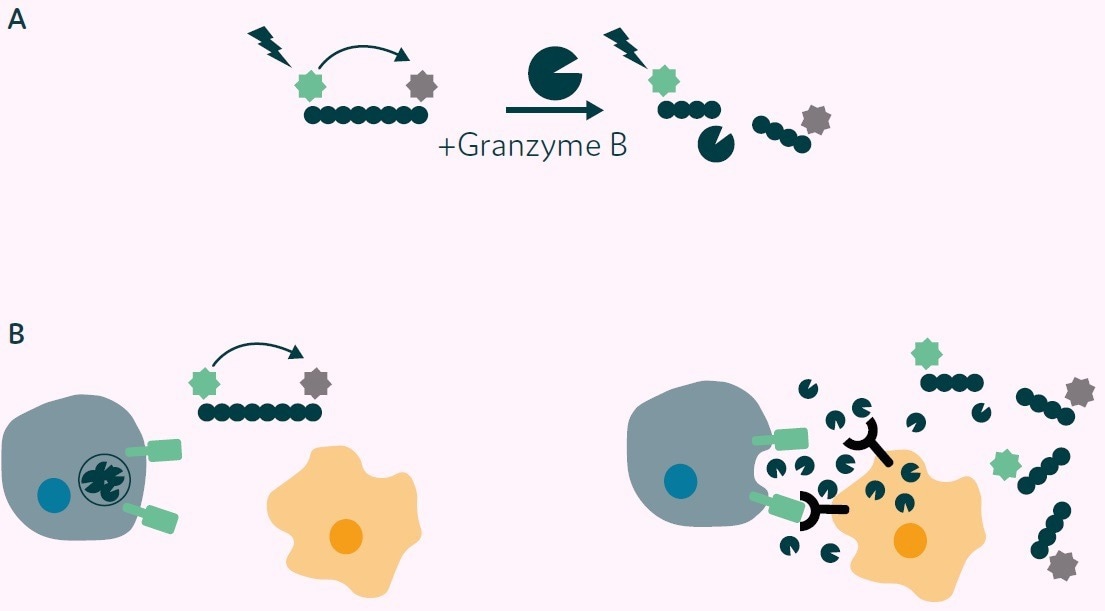
Figure 2. Principle of Granzyme B detection assay. A) Granzyme B substrate peptide labelled with 5-FAM (green star) and a QXL®-520 fluorophore quencher (grey star). No fluorescence is detected on excitation due to Fluorescence Resonance Energy Transfer (FRET) from 5-FAM to QXL®-520 (left side). On cleavage with Granzyme B the quenching molecule is removed, and fluorescence is emitted from 5-FAM (right side). B) Application of Granzyme B activity assay to CAR-T cell/target cell interaction. Left: CAR-T cell and nontarget cell, no CAR mediated signal, no Granzyme B release, 5-FAM fluorescence quenched. Right: CAR-T cell interaction with target cell results in CAR mediated signal, Granzyme B release, substrate peptide cleavage, release of the quencher and fluorescence is then detected. Image Credit: Fluidic Sciences and Sphere Bio
Granzyme B detection in picodroplets
To verify the general feasibility of the assay, two populations of picodroplets of approximately 450 pL volume were generated using the Picodroplet Single Cell Encapsulation System.
One population only consisted of the fluorogenic Granzyme B substrate peptide (serving as a negative control), while the other population co-encapsulated recombinant Granzyme B and the substrate peptide. Figure 3 illustrates micrographs of the two populations in brightfield (left) and green fluorescence (right) after a 2-hour incubation at 37 °C.
Only picodroplets that contained both Granzyme B and the substrate peptide (bottom) exhibited detectable fluorescence after the 2-hour incubation, while picodroplets containing only the substrate peptide (top) displayed low to no background fluorescence.
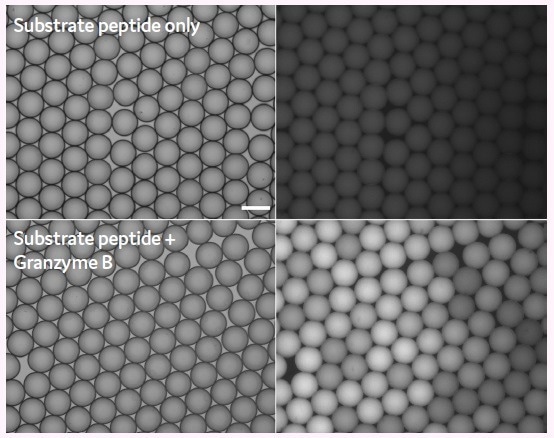
Figure 3. Detection of Granzyme B activity in picodroplets using a fluorogenic 5-FAM/ QXL®-520 substrate peptide. Brightfield (left side) and Fluorescence micrographs (right side) 2h post generation. Scale bar: 100 μm. Image Credit: Fluidic Sciences and Sphere Bio
Granzyme B detection assay with T cells and target cells
In the next step, researchers co-encapsulated polyclonal human donor T cells genetically modified to express a Chimeric Antigen Receptor (CAR) directed against Prostate-Specific Membrane Antigen (PSMA) with target cells expressing PSMA (PC3-LN3-PSMA) and the fluorogenic Granzyme B substrate peptide.
The researchers employed a custom-made biochip with two separate aqueous inlets to keep the CAR T and target cells apart until immediately before encapsulation. The cell concentrations were adjusted so that only 20% of the picodroplets contained a CAR T cell, with over 75% of the picodroplets receiving at least one target cell.
Approximately 15% (0.75 x 0.2) generated picodroplets contained at least one CAR-T cell and one target cell, meaning that 150,000 of the 1 million picodroplets had both a CAR-T cell and a target cell.
As a control, the researchers co-encapsulated the CAR T cells with PC3-LN3 cells lacking PSMA expression, along with the fluorogenic peptide substrate, under otherwise identical conditions.
The picodroplets were collected in an Eppendorf tube and observed under a fluorescence microscope for Granzyme B activity after 2, 4, and 24 hours of incubation.
Figure 4 displays fluorescent micrographs of picodroplets that contain either non-expressing control cells (top row) or PC3-LN3-PSMA target cells (bottom row) and were imaged at 2, 4, and 24 hours (left to right).
The picodroplets that contain PC3-LN3 control cells show minimal Granzyme B activity after 2 to 4 hours. However, multiple fluorescent picodroplets in the picodroplets containing PC3-LN3-PSMA cells are indicative of Granzyme B being released by the CAR-T cells in response to the target cells.
Increased fluorescence is only observed in the negative control after 24 hours of incubation, which may be attributed to either unspecific stimulation or Granzyme B leakage from dead or apoptotic CAR-T cells.
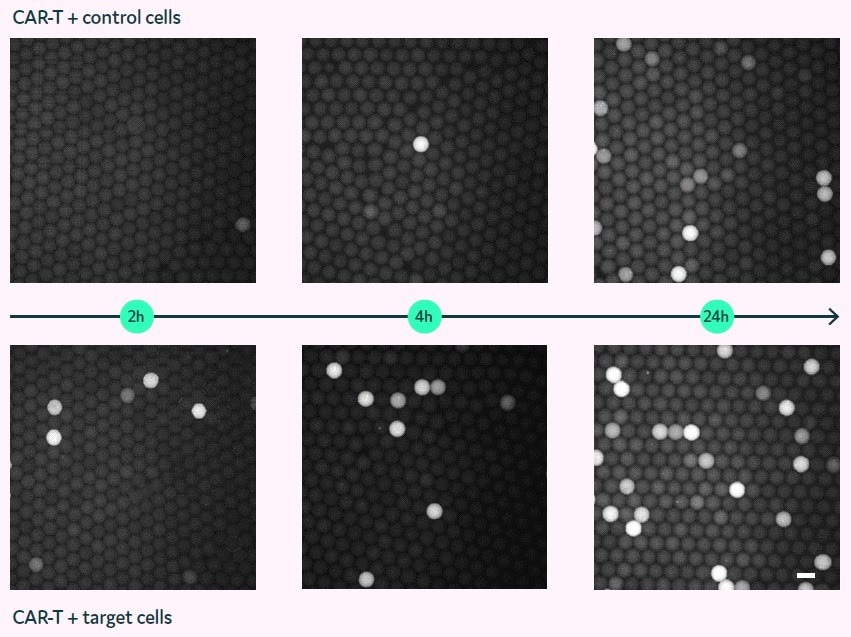
Figure 4. Granzyme B assay in picodroplets with co-encapsulated CAR-T and target cells (bottom) or control cells (top). Fluorescence micrographs of picodroplets after 2, 4, and 24 h (left to right). Scale bar: 100 μm. Image Credit: Fluidic Sciences and Sphere Bio
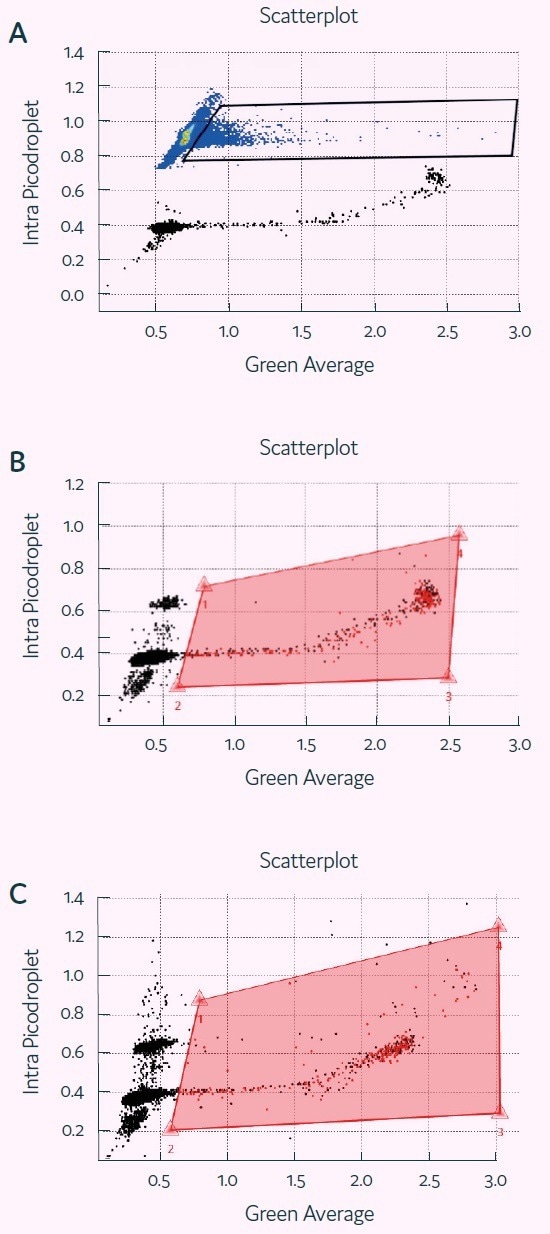
Figure 5. Detection of Granzyme B activity in picodroplets with Cyto-Mine®. Screenshots of Cyto-Mine® software during detection/ sorting, showing scatterplot of picodroplet size (Intra picodroplet, ms) against Fluorescence signal (Green Average, V) at t=0 (A), 1h (B) and 2h (C). The middle and bottom panel also show polygon gates used for determining percentage of positive picodroplets and for sorting. Image Credit: Fluidic Sciences and Sphere Bio
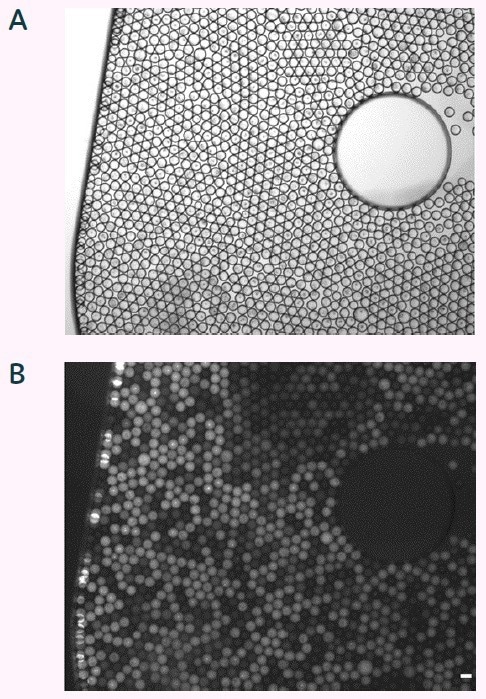
Figure 6. Brightfield (A) and fluorescent micrograph (B) of sorted picodroplets in dispensing chamber. Scale bar 100 μm. Image Credit: Fluidic Sciences and Sphere Bio

Figure 7. Cyto-Mine® - Sphere Fluidics’ automated and fully integrated single cell analysis system. Image Credit: Fluidic Sciences and Sphere Bio

 Download the full paper
Download the full paper
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.