The increase in the utilization of monoclonal antibodies as therapeutics over the past two decades has been remarkable, with over 80 mAbs approved for use.
The quick pace of clinical studies and approvals in the antibody-based therapeutics sector is generating a significant demand for biopharmaceutical companies to shorten the time it takes to bring new biologics to market.
Traditional methods for screening large cell panels used in antibody discovery during lead generation are demanding in terms of resources and labor, requiring multiple pieces of equipment for each step, such as single-cell analysis, sorting, imaging, and dispensing into individual wells of microtiter plates, resulting in major bottlenecks in identifying lead candidates.
Cyto-Mine® helps to address the time-consuming, labor-intensive nature of screening large cell populations for rare antigen-specific, antibody-secreting cells during antibody discovery by streamlining the traditional multi-step process into one seamless, fully-integrated one-day workflow.
Hybridoma-based antibody discovery
The discovery of antibodies often begins with the identification of a known, validated target. The next step is to generate candidate antibodies for hit selection.
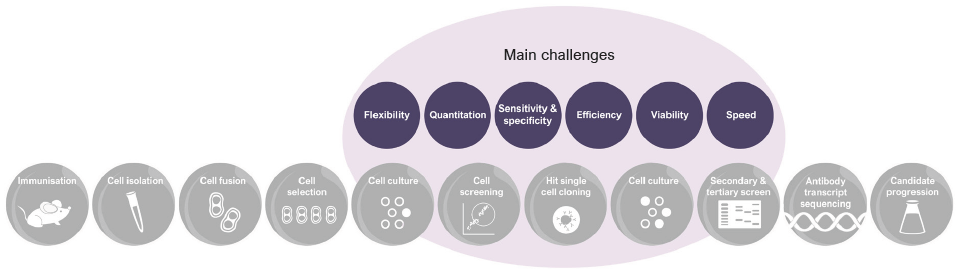
Figure 1. Traditional workflow in hybridoma screening. Image Credit: Fluidic Sciences and Sphere Bio
Historically, immunization of animals, such as mice, rats, or rabbits, is used to induce an immune response. After several weeks, B-cells can be harvested and merged with myeloma cells to form hybridoma populations (Figure 1).
An alternative approach involves directly isolating B-cells. Regardless of the method used, extensive screening and characterization of the cells producing the antibodies are necessary. However, conventional screening methods, such as flow cytometry and ELISA, can result in bottlenecks.
While flow cytometry is a high-throughput method for screening large cell populations, it has several limitations.
The harsh process of flow cytometry can cause shear stress that can damage cells, including rare or valuable ones, thereby decreasing their viability and potential for recovery.
Additionally, flow cytometry is not an effective method for measuring cell secretion. Cold capture secretion assays can retain secreted molecules at the cell surface through cell manipulation, but any uncaptured molecules will spread into the extracellular environment, resulting in an inaccurate representation of cell secretion.
Once the antibodies have been screened and characterized, they must be subcloned into monoclonal populations.
This process involves the use of semi-automated technologies, such as cell printers and cell-in-well imagers, which increase the number of instruments involved in the workflow, making it more time-consuming and labor-intensive.
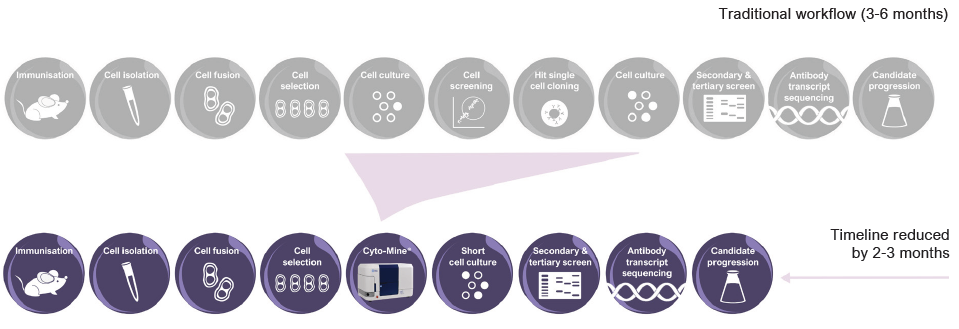
Figure 2. Accelerated workflow in hybridoma screening. Image Credit: Fluidic Sciences and Sphere Bio
Cyto-Mine® is a fully integrated platform that uses microfluidic picodroplet technology to streamline the discovery and development of antibody-based therapeutics.
By combining cell isolation, assay, sorting, imaging, and dispensing into a single automated workflow, Cyto-Mine® reduces the timeline for antibody discovery (as shown in Figure 2).
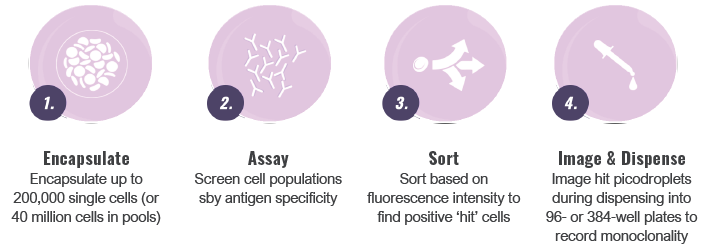
Image Credit: Fluidic Sciences and Sphere Bio
The platform addresses the major challenges in the antibody discovery workflow, including:
- Flexibility: Adaptable assay design for specific needs
- Measurement: Semi-quantitative assays of antibody secretion
- Sensitivity and Specificity: Detect antibodies of interest
- Efficiency: Screen the entire cell population
- Viability: Maintain high levels of cell viability
- Speed: Reduce total workflow timelines
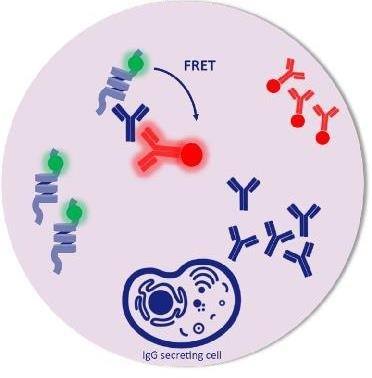
Picodroplet. Image Credit: Fluidic Sciences and Sphere Bio
Assay flexibility
Fluidic Sciences and Sphere Bio provides antigen-specific assays for the detection of specific targets. The company offers flexible assay design, including the capability to create custom-made assays to meet specific application requirements. These assays can include antigen-specific assays for hybridoma screening or B-cell mining.
Antibody measurement
Picodroplet-based technologies offer a highly sensitive way to assay and select antigen-specific, antibody-secreting cells that conventional methods cannot.
To demonstrate the semi-quantitative nature of the Cyto-Mine® antigen-specific assay, experiments were performed using human TNF-alpha as a model antigen. The results, shown in Figure 3, illustrate how different concentrations of the antigen were resolved into distinct populations.
As a control, the samples were analyzed along with control IgG that did not express the antigen using a fluorescent plate reader (Figure 4).
The titration curve in Figure 4 demonstrates that the antigen-specific assay probes only detected the human TNF-alpha population and not the control IgG, indicating that the assay is specific to the antigen of interest.
Finally, in Figure 5, a hybridoma population secreting human TNF-alpha-specific IgG at unknown concentrations was analyzed to show the spread of production capacities among the hybridoma population and to demonstrate that the assay reagents could identify secreted antigen-specific antibodies.
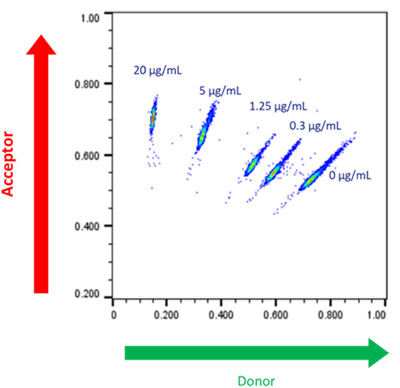
Figure 3. Cyto-Mine® Scatter Plot. A library of picodroplets was made containing different concentrations of target anti-human TNF-alpha IgG and detected using an antigen-specific assay. Image Credit: Fluidic Sciences and Sphere Bio
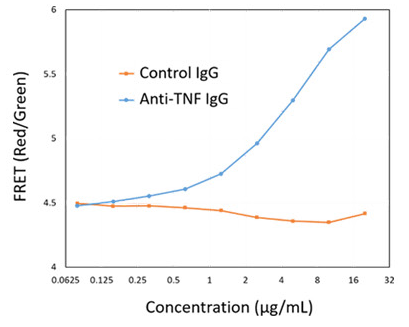
Figure 4. Representative Titration Curve. Generated using the Cyto-Mine® antigen-specific assay. In this example, the control IgG confirms specificity of the assay for human TNF-alpha. Image Credit: Fluidic Sciences and Sphere Bio
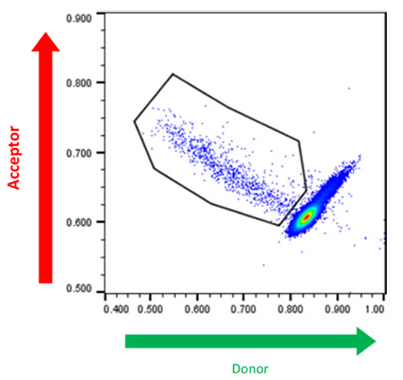
Figure 5. Cyto-Mine® Scatter Plot. FRET signal generated from hybridomas encapsulated in picodroplets and screened for secretion of human TNF-alpha specific IgG. Image Credit: Fluidic Sciences and Sphere Bio

 Download the full paper
Download the full paper
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.