Advancement of therapeutic antibody discovery faces time and economic constraints. Merck R&D experts address this issue using Fluidic Sciences and Sphere Bio's microfluidic picodroplet technology.
Fast, high-throughput screening of primary antibody-secreting cells from rodent and human donors demonstrates precise antibody binding to recombinant antigens, highlighting the optimized and automated process of Fluidic Sciences and Sphere Bio’s Cyto-Mine® and Cyto-Mine® Chroma platforms in antibody discovery projects.
Introduction
The US Food and Drug Administration (FDA) approved muromonab-CD3, the first therapeutic monoclonal antibody (mAb), for clinical usage in 1986.1 Since then, more than 190 therapeutic antibody medicines have been approved globally, with hundreds more presently being researched.2
Traditional mAb production approaches, such as hybridoma technology and display methods, have limited throughput, are time-consuming, and require significant resources.3
Modern display approaches provide increased immunological diversity but involve tedious subcloning of surface-displayed hits into soluble formats, resulting in a loss of diversity in the final library.4
Both of these techniques do not require the knowledge of a target’s structure for antibody development, resulting in downstream ambiguity regarding safety.5
Compared to these constraints, droplet-based microfluidics technology has the distinct benefit of encapsulating individual antibody-secreting cells (ASCs) in miniature water-in-oil droplets with remarkable throughput.
Cell encapsulation in droplets creates a protective environment that maximizes cell viability and test sensitivity for various cell types, including primary B cells from human and animal donors.
Antibodies produced by droplet-encapsulated ASCs maintain their genotype-phenotype linkage as well as native chain pairing, which can be evaluated using next-generation sequencing (NGS) and employed for direct antibody subcloning or library formation.
As a result, droplet-based microfluidic technologies, like the Cyto-Mine®, are creating new opportunities for identifying antibodies with novel functional characteristics or against challenging targets, such as insoluble membrane proteins.6-8
Cyto-Mine®, which uses picodroplet-based technology, can effectively isolate and evaluate up to 40 million cells with antigen specificity in one day by encapsulating them in individual picodroplets, testing for the target antigen, choosing rare antigen-specific ASCs, and dispensing them as single cells into 96- or 384-well microtiter plates (MTPs) for downstream functional analysis.
This article shows how Merck’s R&D experts used Cyto-Mine® to quickly identify target-specific antibodies from B cells isolated from human or rodent donors and tested for antibody secretion, specific binding to recombinant antigens or antigen-positive cells, and functional investigations.9
Materials and methods
Plasma cell preparation
Human plasma blasts were extracted from the peripheral blood of healthy donors 6 to 7 days after tetanus toxoid (TT) vaccination. Peripheral blood mononuclear cells (PBMCs) were isolated according to the SepMate™-50 IVD (StemCell) manual.
The EasySep™ human Pan-B cell enrichment kit (StemCell) and a RoboSep device (StemCell) were used to enrich Pan-B cells in PBMCs.
CD38+ cells were then isolated by using a CD38 monoclonal antibody (HIT2), Biotin, eBioscience™ (Invitrogen), in a dose of 0.5 µg/100 µl, in conjunction with EasySep™ human biotin positive selection kit II (StemCell).

Image Credit: Fluidic Sciences and Sphere Bio
Primary mouse plasma cells from bone marrow, lymph nodes, and splenocytes were generated five days after being boosted with a recombinant C-terminal domain tetanus toxoid fragment (TT-CTD) or an extracellular domain of a Cancer-Related Antigen (CRA).
The EasySep™ Mouse CD138 Positive Selection Kit (StemCell) enriched cells for ASCs utilizing a RoboSep device (StemCell). Ultimately, isolated human CD38+ or murine CD138+ cells were moved to fresh cell media before being processed for Cyto-Mine® evaluation.
Plasma cell screening with Cyto-Mine®
To separate plasma cells that secrete antigen-specific antibodies, 1 million ACSs were resuspended in 1 mL of the encapsulation media, as displayed in Table 1.
In the FRET-based investigation, which is used to test for antibody secretion and recombinant antigen binding, AlexaFluor488 labeled TT-CTD and Goat F(ab’)2 Anti-Human IgG-(Fab’)2 (DyLight-594) were incorporated as FRET donor and acceptor, respectively, to reach 1 mL of the basic encapsulation medium for testing human CD38+ cells secreting antibodies against TT (Figure 1).
To separate CD138+ murine plasma cells that secrete antibodies against biotinylated CRA, cells were resuspended in 1 mL of encapsulation medium with Streptavidin-AF488 labeled with biotinylated CRA and Goat F(ab’)2 Anti-Mouse IgG – (Fab’)2 (DyLight® 594) as FRET donor and acceptor, respectively.
Table 1. Composition of the picodroplet encapsulation medium. Source: Fluidic Sciences and Sphere Bio
| Encapsulation medium component % |
% (v/v) |
| Cell culture medium |
83% |
| OptiPrep™ Density Gradient Medium |
16% |
| Pluronic™ F-68 (10%) |
1% |
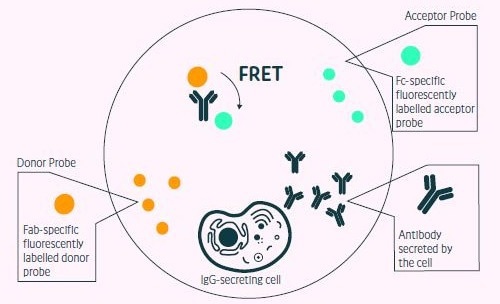
Figure 1. Positive events readout using the Cyto- Mine® picodropletbased FRET IgG secretion assay. This model shows the standard assay to screen for IgG secreting cells. A customized pair of IgG-specific fluorescent probes are trapped within each picodroplet during encapsulation. IgG secreted from the encapsulated cell is recognized by the detection probe pair forming a 3-body FRET complex that induces a shift in fluorescent signal. Image Credit: Fluidic Sciences and Sphere Bio
Cyto-Mine® process for sorting ASCs for antigen-specificity
The Cyto-Mine® process automates single-cell encapsulation, incubation, screening, sorting, isolation, and validation of target-specific ASCs (Figure 2).
To encapsulate 1 million ASCs, the cell solution was filtered through a 40 µM cell strainer and pipetted into a Cyto-Cartridge®, which was then loaded into the Cyto-Mine®.
Cells in 1 mL were encapsulated in 2 million 450 pL droplets and immediately incubated in situ at 37 °C to enable antibody production within the picodroplets to reach a measurable level, allowing the FRET signal to form.
The compact format allows for an ultrasensitive, quick assay that takes around 0.5 to 2 hours, based on the cell population’s production rate.
Cyto-Mine® assessed each picodroplet’s fluorescence, and, similar to flow cytometry, antigen-positive hits were collected and stored in a refrigerated microchamber before dispensing.
Before dispensing, the Cyto-Mine® imaged each droplet five times in a row to provide visual proof of a single-cell progenitor in individual wells of 96- or 384-well MTPs prefilled with the lysis buffer.
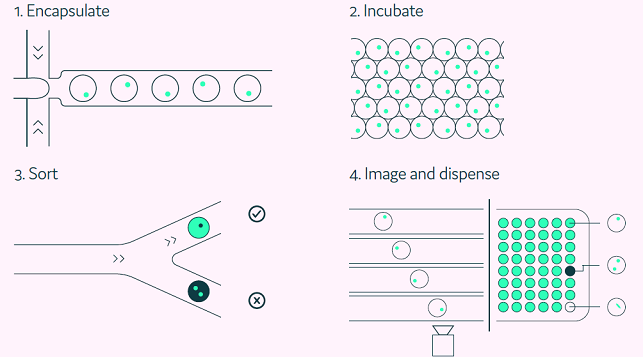
Figure 2. The Cyto-Mine® workflow integrates the screening, sorting, isolation and verification of antigen-specific clones into a fully automated process. Image Credit: Fluidic Sciences and Sphere Bio
Results
Screening for human ASCs using Cyto-Mine®
As a demonstration of Cyto-Mine’s high throughput and capability to mine plasma blasts for human diversity, three million ASCs extracted from the peripheral blood of TT-vaccinated human donors were tested for TT-antibody-specific secretion either on the same day of procuring or frozen for five days before testing with Cyto-Mine® (Figure 3A and B, respectively).
One thousand droplets were sorted for antigen-specificity, and 433 were dispensed as single cells into final MTPs. Single-cell PCR analysis was used to retrieve IgG sequences, and specific binding was validated with biolayer interferometry (BLI) (Figure 3C).
The downstream evaluation showed that the Cyto-Mine®-based antibody discovery campaign effectively produced a panel of 44 varied sequences and high affinity completely human TT antibodies from the plasma blasts enriched from the peripheral blood of a TT-vaccinated human donor in less than one month (Table 2).
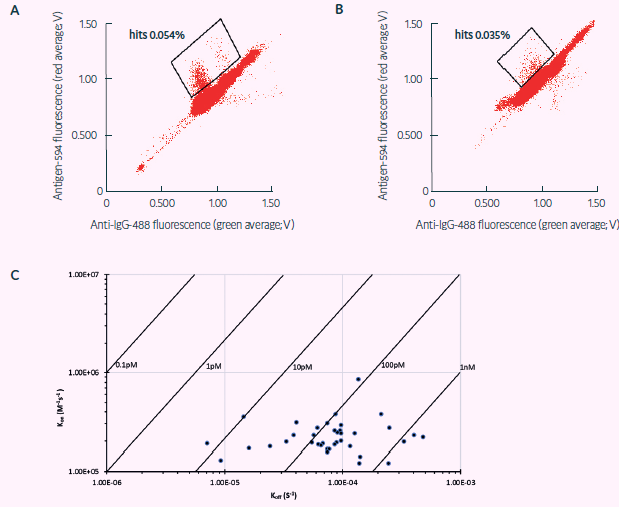
Figure 3. Sorting of freshly sourced and revived human ASCs for TT target specificity (adapted from Gaa et al., 2021, under CC BY 4.0 License). Sorting Scatter plot obtained from, A) human ASCs, and B) revived human plasmablasts secreting antibodies against TT via FRET: a decrease in green and an increase in red fluorescence indicates FRET by recombinant target binding in the respective droplets. C) TT binding affinities via BLI showing mainly subnanomolar TT affinities of binders obtained via Cyto-Mine® within 4 weeks from ASC sourcing. Image Credit: Fluidic Sciences and Sphere Bio
Screening of rodent ASCs using Cyto-Mine®
To illustrate the usefulness of a comparable streamlined methodology for measuring immunological diversity in rodents, ASCs obtained from rodents pre-immunized with a CRA were screened using the same method.
Cyto-Mine®’s picodroplet technology made encapsulating CD138+ plasma cells in droplets easier, which were subsequently tested for antigen specificity at the single-cell level (Figure 4). Of the droplets examined, 2,300 contained antigen-specific ASCs.
From these, 635 were selected for further downstream examination. This procedure yielded 98 complete monoclonal antibody gene PCR amplicons, which were then subcloned and produced. Thirty of these antibodies were confirmed to be target-specific (Table 2).
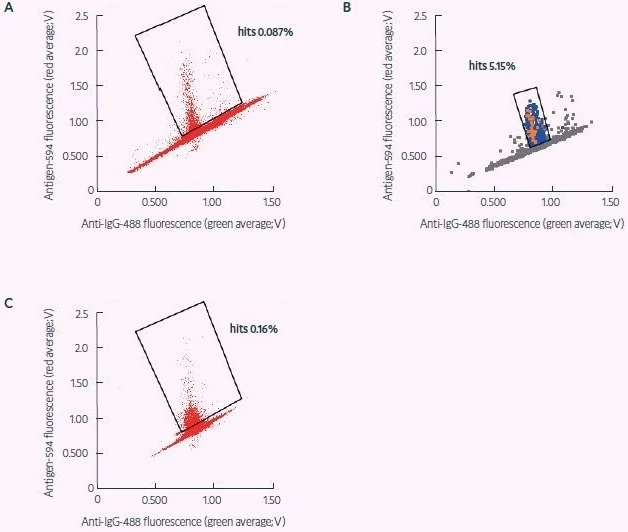
Figure 4. Sorting of fresh and revived murine ASCs for CRA target specificity (adapted from Gaa et al., 2021, under CC BY 4.0 License). A) Sort of freshly obtained murine plasma cells for secreted antibodies specific for a CRA. B) Dispensing of sort shown in (A) – binders later confirmed as positive are shown in red, dispensed droplets in light blue, and non-dispensed droplets in gray that were used as a negative reference signal during dispensing. Positions of positive signals confirm appropriate gating. C) Sort for CRA specificity as in (A), applying frozen and revived plasma cells. The statistics of these antibody hit discovery campaigns are shown in Table 2. Image Credit: Fluidic Sciences and Sphere Bio
Table 2. Screening of human and mouse ASCs for target specificity by Cyto-Mine® (adapted from Gaa et al., 2021, under CC BY 4.0 License). Statistics for two Cyto-Mine® antibody discovery campaigns. Magnetic-activated cell sorting (MACS) output in the TT campaign represents CD38 + enriched plasma blasts obtained from 297 mL of one healthy vaccinated donor’s peripheral blood, whereas CRA campaign MACS output originates from pooled bone marrow, lymph node, and spleen samples of two immunized wild-type mice enriched for CD138+ plasma cells. Of 1050 and 1354 CRA-specific droplets dispensed, 635 and 96 were analyzed and yielded 98 and 33 full monoclonal antibody (mAb) gene PCR amplicons, respectively. 33 of 2-month campaign were not further processed. The difference between the number of sorted and dispensed cells arises from the amalgamation of positive and negative droplets during sorting to fulfil a droplet requirement. Despite implementing gating strategies for consistency, divergences in photomultiplier tube settings introduce variations between the sorting and dispensing modes. Source: Fluidic Sciences and Sphere Bio
| Antigen |
TT |
CRA |
| ASC Source |
Freshly prepared |
5 days Freeze/thaw |
Freshly prepared |
5 days Freeze/thaw |
| MACS output |
5,230,000 |
6,520,000 |
| Viability (5) |
98 |
99 |
95 |
92 |
| Percentage ASCs/Total cells (%) |
n.d. |
n.d. |
11 |
9 |
| Number of droplets/cells |
2*106 /2*106 |
1*106 /1*106 |
2*106 /1*106 |
2*106 /1*106 |
| Number of cells sorted |
823 |
221 |
~2,300 |
~3,000 |
| Number of cells dispensed |
318 |
117 |
635 |
96 |
| Full mAb gene PCR amplicons yielded |
83 |
47 |
98 |
33 |
| Number of mAbs produced |
72 |
92 |
n.d |
| Number of targetspecific mAbs |
44 |
30 |
n.d |
n.d., not determined
Conclusions
These results demonstrated the efficacy of Cyto-Mine® in screening millions of primary B cells, allowing Merck R&D experts to develop highly specific TT and CRA antibodies.
The antibody discovery process took only 28 days, with Cyto-Mine® employed on day one for quick screening, isolation, and single-cell analysis of antigen-specific B cells. This was followed by 27 days for gene synthesis, single-cell cloning, generation, and validation.
Compared to conventional B cell mining processes, Cyto-Mine® enables high throughput screening on the same day as cell isolation.
Antigen-specific B cells are often extracted using flow cytometry.10 However, flow cytometry can be extremely harsh on cells, resulting in considerable cell loss, and is confined to membrane-bound antibodies.
Water-in-oil picodroplets offer a unique protective environment for cell viability and integrity because the aqueous phase is made up of a specific cell-grown medium, and the proprietary Cyto-Mine® surfactant formulations protect them from shear stress as they flow through the microfluidic channels. Therefore, Cyto-Mine®’s picodroplet-based method is gentle on the chosen cells.
In addition to the single well export of the Cyto-Mine® screening workflow shown in this article, Cyto-Mine®’s picodroplet-based technology enables bulk dispensing for probable re-compartmentalization for NGS evaluation of IgG repertoire analysis, enabling the examination of both a spatial and temporal immune response.
References
- Kuhn, C. and Weiner, H.L. (2016). Therapeutic anti-CD3 monoclonal antibodies: from bench to beside. Immunotherapy, 8(8), pp. 889-906
- Antibody Society. Antibody therapeutics product data. Available at: https://www. antibodysociety.org/antibody-therapeuticsproduct-data/ [Accessed 3 October 2023]
- Mitra, S. and Tomar, P.C. (2021). Hybridoma technology; advancements, clinical significance, and future aspects. Journal of Genetic Engineering & Biotechnology, 19(1), p. 159
- Ministro, J., Manuel, A.M. and Goncalves, J. (2020). Therapeutic Antibody Engineering and Selection Strategies. Advances in Biochemical Engineering/ Biotechnology, 171, pp. 55-86
- Lausten, A.H. et al. (2021). Animal Immunization, in Vitro Display Technologies, and Machine Learning for Antibody Discovery. Trends in Biotechnology, 39(12), pp. 1263-1273
- Gérard, A. et al. (2020). High-throughput single-cell activity-based screening and sequencing of antibodies using droplet microfluidics. Nature Biotechnology, 38(6), pp. 715-721
- Gaa, R. Qingyong, J. and Doerner, A. (2023). Antibody-Secreting Cell Isolation from Different Species for Microfluidic Antibody Hit Discovery. Methods in Molecular Biology (Clifton, N.J.), 2681, pp. 313-325
- Liu, X. et al. (2016). High-throughput screening of antibiotic-resistant bacteria in picodroplets. Lab on a Chip, 16(9), pp. 1635-1643
- Gaa, R. et al. (2021). Versatile and rapid microfluidics-assisted antibody discovery. mAbs, 13(1), 1978130
- Boonyaratanakornkit, J. and Taylor, J.J. (2019). Techniques to Study Antigen-Specific B Cell Responses. Frontiers in Immunology, 10, 1694
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.