This article will explore how Cyto-Mine® can be utilized for the analysis of large heterogeneous cell populations on a cell-by-cell basis by employing a homogeneous, miniaturized, animal origin-free (AOF) IgG secretion assay to measure and detect the greatest antibody-producing clones.
The main aim of Bioprocess Cell Line Development is to achieve high product yields from well-defined and robustly-growing clonal cell lines within weeks instead of months.
A significant bottleneck in this process, however, is the screening and isolation of rare cells with the desired characteristics.
Conventionally, this is accomplished using the resource-intensive technique of limiting dilution, which has been recently assisted by the use of semi-automated technologies, including colony picking, cell sorting, and cell-in-well imagers.
The Cyto-Mine® Single Cell Analysis and Monoclonality Assurance System help to address the challenges faced by current technologies through the screening of hundreds of thousands of individual cells for the secreted target protein.
This is followed by isolating and dispensing the greatest producers with high viability to microplate wells, as shown in Figure 1.
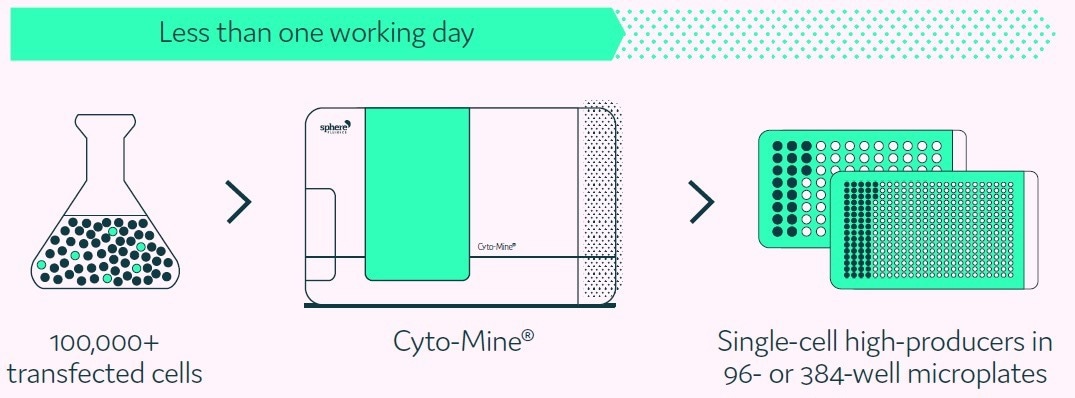
Figure 1. Cyto-Mine® proprietary technology finds and isolates high-producer clones from complex cell populations. Image Credit: Fluidic Sciences and Sphere Bio
The study outlined in this article illustrates the process by which the Cyto-Mine® IgG secretion assay measures the production rate of hundreds of thousands of single cells encapsulated in highly consistent picoliter ‘test tubes.’
Methods
Cyto-Mine® workflow
Cyto-Mine® follows the processes of limiting dilution and productivity screening but in a much more efficient, higher throughput, and fully automated manner.
Using Poisson distribution statistics, cells are encapsulated into 300 pL picodroplets of the preferred culture medium at a dilution level that optimizes the number of picodroplets containing only a single cell.
Following this, cells are incubated to allow the secreted target protein to collect inside the picodroplet, which is captured and identified using the AOF IgG detection reagent that is present in the culture medium. The miniaturized scale means that secreted IgG can be quantified after just 0.5 to 2 hours.
The highest-secreting single cells are consequently sorted for collection. Figure 2 presents a summary of the integrated stages of the Cyto-Mine® process.
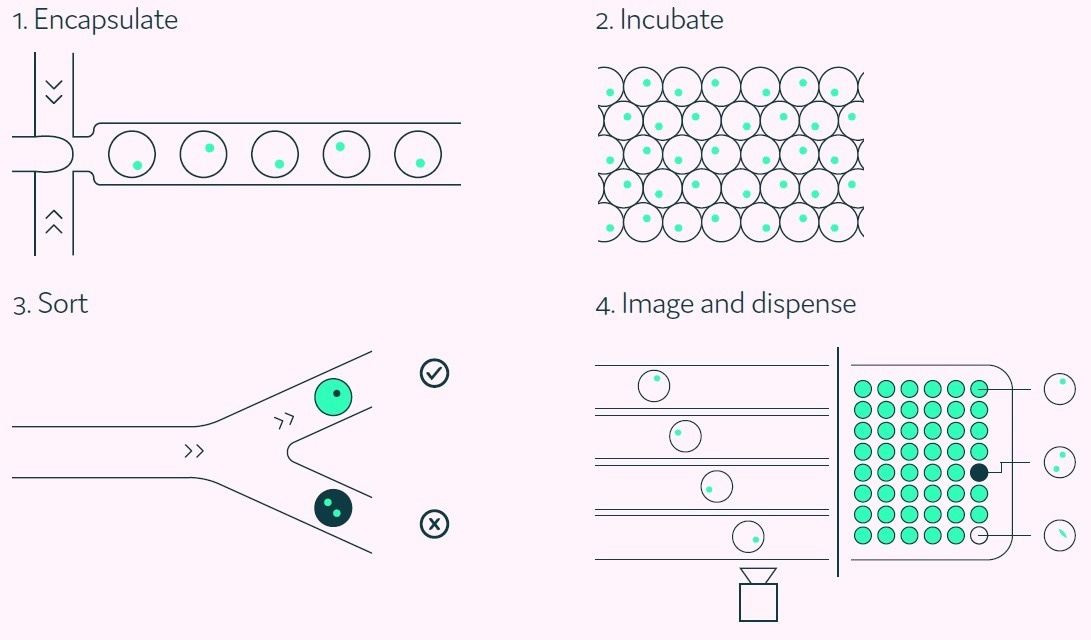
Figure 2. A Cyto-Mine® workflow. Integration of single cell screening, sorting, isolation, and verification using a fully integrated microfluidic process. Image Credit: Fluidic Sciences and Sphere Bio
Cyto-Mine® IgG secretion assay
A main supporting element of Cyto-Mine® is its capacity for measuring the specific IgG production rate of every single cell.
The starting cell population does not demand any prior modification and is simply mixed with the preferred AOF detection reagent before being loaded onto Cyto-Mine®.
The IgG that is secreted by the cell collects within the picodroplet during the in situ incubation stage.
The detection probes bind to the secreted IgG, and this prompts a FRET-mediated shift in fluorescence, as displayed in Figure 3. Subsequently, Cyto-Mine® measures the produced fluorescent signal and converts this to a quantitative output.
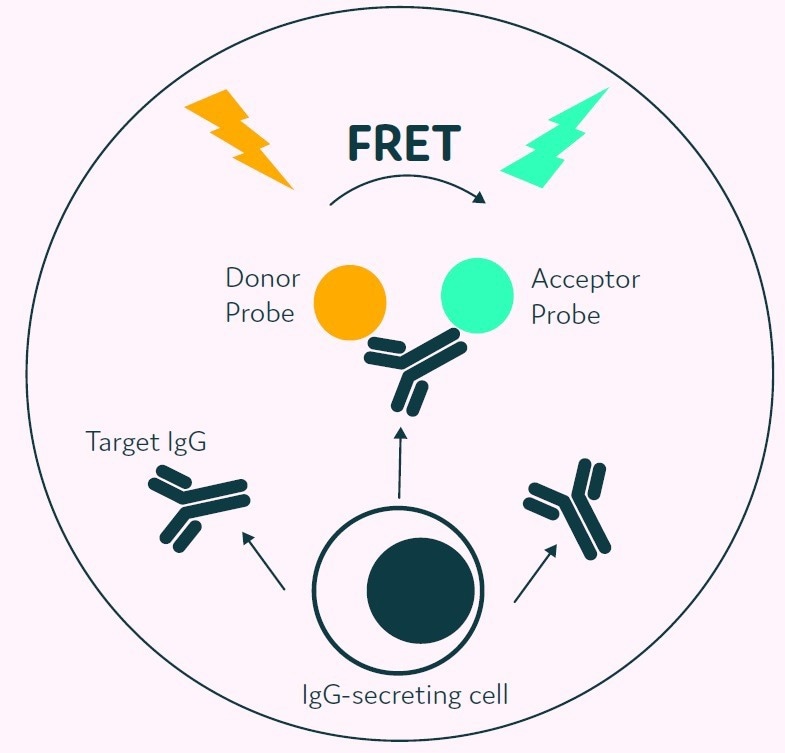
Figure 3. The Cyto-Mine® picodroplet-based IgG secretion assay. A customized pair of IgG-specific AOF fluorescent probes are trapped within each picodroplet. IgG secreted from the encapsulated cell is recognized by the detection probe pair forming a 3-body FRET complex that induces a fluorescent signal. Image Credit: Fluidic Sciences and Sphere Bio
Results
IgG standard titration curve
To validate the Cyto-Mine® IgG secretion assay, five separate populations of picodroplets were produced from a culture medium spiked with human IgG over concentrations ranging from 0 to 20mg/L.
Following this, the five populations of picodroplets were pooled together and analyzed with Cyto-Mine® AOF technology.
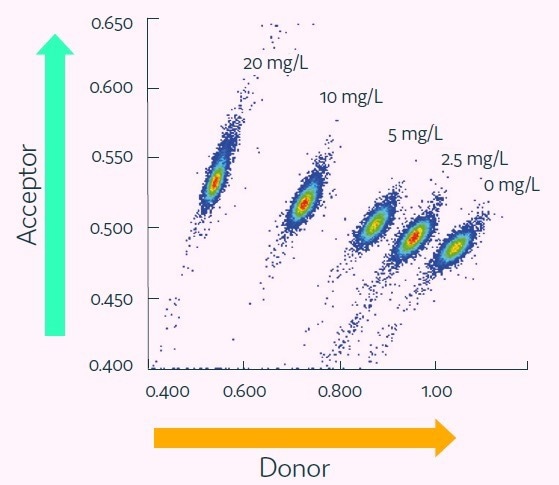
Figure 4. Cyto-Mine® Scatter Plot. Large numbers of individual picodroplets were loaded with the indicated concentrations of human IgG and then resolved using Cyto-Mine® AOF IgG secretion assay and analysis. Image Credit: Fluidic Sciences and Sphere Bio
Figure 4 demonstrates how the various titers resolved into discrete populations.
IgG concentrations up to 20 mg/L represent the typical Cyto-Mine® working range, equivalent to a specific productivity (Qp) of up to 144 pg/cell/day, assuming a one-hour period of incubation. Figure 5 presents a standard titration curve produced using Cyto-Mine® Scatter Plot data.
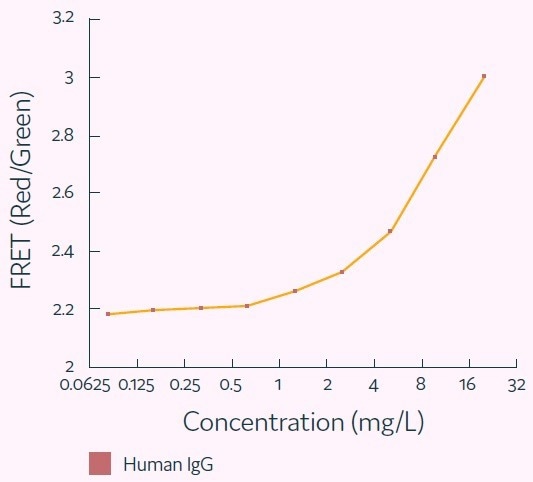
Figure 5. Standard human IgG titration curve derived from Cyto-Mine® AOF IgG secretion assay and analysis. Image Credit: Fluidic Sciences and Sphere Bio
Screening a CHO cell population for high-producers
A heterogeneous pool of CHO cells was stably transfected to express human IgG. This was then mixed with Cyto-Mine® human IgG-specific AOF detection reagent.
The cells were encapsulated into picodroplets and subsequently incubated for two hours. After this, they were analyzed utilizing the Cyto-Mine® IgG secretion assay.
The results demonstrate a population of cells with a high acceptor-to-donor fluorescence ratio and gated for collection.
The bright oval-shaped cluster to the lower-right represents the bulk of data points, comprised of picodroplets containing low- or non-producing cells and empty picodroplets.
The gating polygon is able to be customized by the user to provide additional flexibility for the targeting of the highest-value cells for collection to microplates.
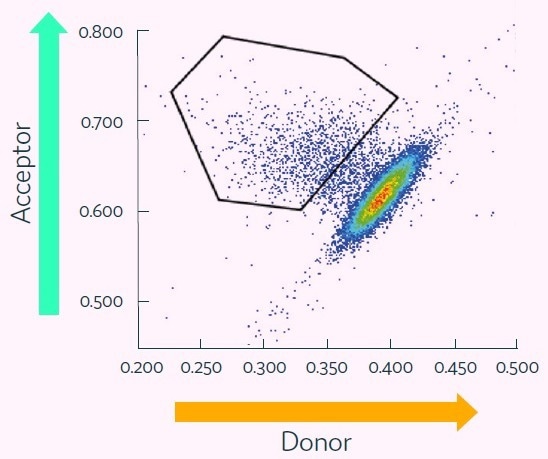
Figure 6. Cyto-Mine® Scatter Plot of FRET signal generated from picodroplet-encapsulated CHO cells incubated with Cyto-Mine® human IgG-specific AOF detection reagent. Image Credit: Fluidic Sciences and Sphere Bio
Conclusions
These results demonstrate the power of Cyto-Mine® for the screening of large cell populations to identify, sort, and isolate high-value clones.
The assay used is quantitative within the range of immunoglobulin typically produced by standard biopharmaceutical production cell types.
The use of Cyto-Mine® and its picodroplet-based IgG secretion assay provides the following key advantages:
- Quality: Unique capability for the mining and isolation of rare, single, viable, high-producing cells
- Sterility: Entirely sterile, disposable, and AOF process
- Simplicity: Seamless, one-step, automated process eliminating the need for multiple instruments
- Efficiency and speed: Allows multiple projects to be run in parallel and frees up time for users to take on other activities
- Traceability: Provides storable images that prove single cell status at the time of dispensing for the required documented evidence of monoclonality
- Miniaturization: Facilitates pL-level high-throughput screening with minimal use of assay reagents

 Download the full white paper
Download the full white paper
Acknowledgments
Produced from materials originally authored by Fluidic Sciences and Sphere Bio. The work detailed in this article was partly funded by the UK Government Advanced Manufacturing Supply Chain Initiative (AMSCI) as part of the BioStreamline Project for the development of new approaches for more efficient development and manufacture of next-generation biologics.
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.