This article discusses how Cyto-Mine® facilitates the mining of rare cells from complex cell populations and subsequent cloning to microplates, with evidence of single-cell status using a seamless, completely automated process.
One of the most significant steps in the submission of a New Drug Application (NDA) for a biologic is proof that the molecule is generated from a clonal cell line. Traditionally, this has been dependent on the demonstration of statistical probability that the cell bank was cloned from a single cell progenitor.
Lately, cell-in-well imagers have gained much interest as a tool for visual verification following cloning by limiting dilution, colony picking, or cell sorting.
Some of the top pharmaceutical companies have made considerable investments in the integration of separate systems into a continuous workflow, while for many, the process of screening, cloning, and verification necessitates several discrete steps involving manual intervention at each stage.
The main limitation of cell-in-well imagers is that only the base of each microplate well is visualized such that cells that have not or cannot settle to the bottom will fail to be detected.
The Cyto-Mine® Single Cell Analysis and Monoclonality Assurance System (as shown in Figure 1) help address the present technologies’ weaknesses by screening hundreds of thousands of individual cells for protein production, specificity, or functionality at the picoliter level.
It subsequently sorts positive hits and carefully dispenses each one individually to microplate wells. Assurance of clonality is achieved via multi-frame imaging of the single status of each cell single status at the time of dispensing.
Cell encapsulation delivers a protective environment that provides high viability and assay sensitivity over a wide range of cell types, including hybridomas, B cells, and CHO cells.
The study described in this article demonstrates the main steps through which Cyto-Mine® identifies, selects, and isolates single, viable, high-value cells with assured clonality.

Figure 1. The benchtop Cyto-Mine® system designed for use in Class II biosafety cabinets. Image Credit: Fluidic Sciences and Sphere Bio
Cyto-Mine® workflow
Cyto-Mine® rivals both limiting dilution and cell screening processes but in a highly efficient and completely automated way. The system’s unique ability to offer high-throughput cell screening and monoclonality assurance is made possible through a series of four seamless steps:
- Encapsulation of cells into picodroplets at extremely high dilution for maximization of the number of picodroplets that contain only one cell.
- In situ incubation of the resulting picoliter ‘test tubes’ to assay the protein secreted by each cell.
- A sorting phase that selectively isolates the highest value cells in picodroplets and diverts all other picodroplets to waste.
- High-speed, multi-frame imaging of each picodroplet immediately before dispensing takes place to deliver visual evidence of single-cell status.
Step 1: Encapsulation of cells into picodroplets
The Cyto-Mine® process is set up by preparing the target cell population to the indicated concentration in the preferred culture medium, mixing it with appropriate detection probes, and then pipetting it into a disposable Cyto-Cartridge® (Figure 2), which is then loaded into Cyto-Mine®.
When the run begins, Cyto-Mine® gently pumps the cell suspension through microfluidic structures, cross-mixing it with the bio-compatible surfactant, CytoSurf®, to encapsulate cells into 300 pL picodroplets of the culture medium, as presented in Figure 3.
Using High Fidelity Mode, the sample dilution is set to 0.05 cells per picodroplet such that ~95% of picodroplets are empty, ~5% contain a single cell, and the probability of 2 or more cells per picodroplet is ~0.1%.

Figure 2. Single-use Cyto-Cartridge®. Image Credit: Fluidic Sciences and Sphere Bio
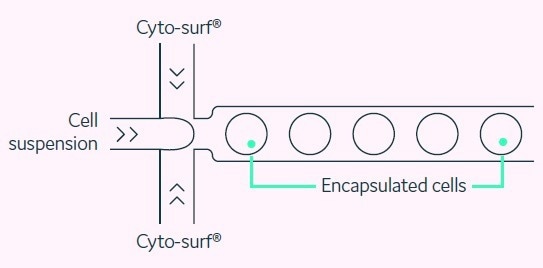
Figure 3. Cell encapsulation. Picodroplets at a uniform size of 300 pL are generated such that only one in 20 contains a cell. Image Credit: Fluidic Sciences and Sphere Bio
Step 2: Incubation and secreted protein assay
Millions of produced picodroplets are collected into a chilled, Peltier-controlled incubation chamber (as displayed in Figure 4), where incubation takes place at up to 37 °C to activate the desired detection assay.
The miniaturized scale means that secreted protein such as IgG from a single cell can be quantified after just 0.5 to 2 hours.
More information regarding the Cyto-Mine® IgG Secretion Assay and the Antigen-specific Assay can be found in Fluidic Sciences and Sphere Bio’ Application Notes 01 and 02, respectively.
Following incubation, the chamber returns to its chilled state to prevent further assay reaction. The picodroplets are subsequently channeled to the sort phase.
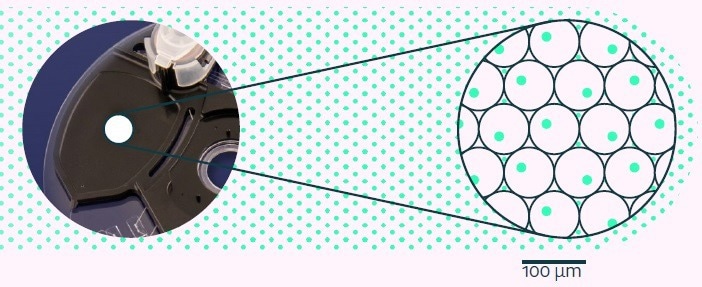
Figure 4. Picodroplets held in the Cyto-Cartridge® incubation chamber are incubated for a defined period to enable the single-cell-based assay to develop. Image Credit: Fluidic Sciences and Sphere Bio
Step 3: Picodroplet sorting
After incubation, Cyto-Mine® sequentially sorts all picodroplets using fluorescence detection, with the positives being actively channeled for collection. The majority of picodroplets, which are either empty or contain low-fluorescing cells, are directed to waste.
Cyto-Mine® can detect the dispersed fluorescence within the picodroplet (e.g., by a secreted protein assay) or the intracellular fluorescence (e.g., by an intrinsic functional assay marker or viability stain ), as displayed in Figure 5.
The user can choose to define and modify the population of cells for collection. The selected picodroplets are subsequently stored in a chilled microchamber before being dispensed.

Figure 5. Options for sorting on Cyto-Mine® by detection of fluorescence either trapped in the picodroplet (A) or intrinsically within the cell inside the picodroplet (B). Image Credit: Fluidic Sciences and Sphere Bio
Step 4: Visual verification and dispensing
After the sorting phase is completed, the selected picodroplets that contain the high-value cells are spaced out, imaged, and dispensed to either 96- or 384-well microplates that are prefilled with an appropriate culture medium, as presented in Figure 6.
The imaging process utilizes an ultra-highspeed, brightfield camera to obtain numerous frames of each picodroplet as it travels through the microfluidic channel immediately before dispensing.
As cells within picodroplets are in constant motion, this multi-frame imaging circumvents the situation where two superimposed cells might be incorrectly detected as one (Figure 7).
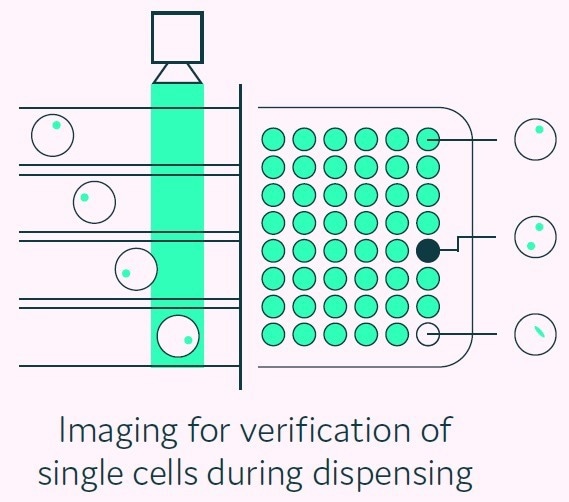
Figure 6. Cyto-Mine® visual verification and dispensing. Immediately prior to dispense, picodroplets are brightfield-imaged to identify and record the number of cells per picodroplet, and also re-measured for picodroplet fluorescence. Image Credit: Fluidic Sciences and Sphere Bio
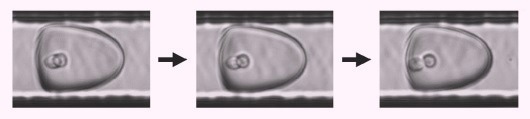
Figure 7. Brightfield multi-frame imaging avoids potential miscounting by detecting encapsulated cells at multiple time points as they rotate into different positions. Image Credit: Fluidic Sciences and Sphere Bio
On-the-fly software analysis can be used to identify and annotate each picodroplet that clearly contains a single cell. Any picodroplets that contain more than one cell, or those exhibiting any ambiguous result, are annotated accordingly on the microplate map (see Figure 8).
Picodroplet fluorescence is measured again when in assay mode, and the reading is documented at the point of dispense.
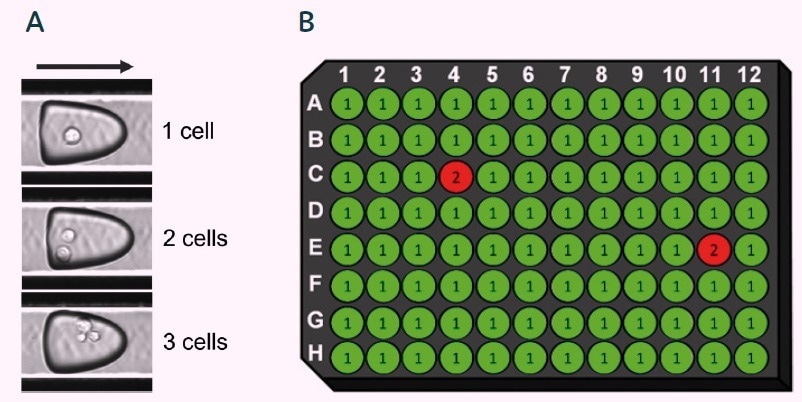
Figure 8. Cyto-Mine® data-tracking and verification of single-cell status. Software analyses and identifies the number of cells per picodroplet (A), and displays the results in microplate map format (B). Only picodroplets that unambiguously contain a single cell are given single-cell status. Fluorescent intensity is also documented for each well (not shown). Image Credit: Fluidic Sciences and Sphere Bio
Cyto-Drop™, the proprietary dispense mechanism of Cyto-Mine®, gently dispenses verified single cells to prefilled microplates. As the picodroplet comes into contact with the culture medium in the microplate well, it dissociates, liberating the cell into the culture medium.
The plate-filling speed is rather quick, taking less than two minutes to fill a 96-well plate and four to six minutes for a 384-well plate. The protective nature of the Cyto-Mine® picodroplet technology and workflow generates clones with strong outgrowth potential.
Conclusions
The four consecutive steps in the fully integrated Cyto-Mine® workflow deliver a powerful yet unique solution to address the challenge of screening vast numbers of cells and subsequently isolating the highest-value clones with confidence that they originate from a single-cell progenitor.
When used in combination with single-use, disposable reagents and consumables, Cyto-Mine® provides the following key benefits:
- Simplicity: A seamless and completely automated process for the screening and isolation of verified clones on a single platform
- Quality: A unique capacity for mining and isolating rare, single, viable, high-producing cells
- Traceability: Provides storable visual evidence of single-cell status at the time of dispensing, which is necessary for the required documented evidence of monoclonality
- Strong outgrowth: Gentle picodroplet encapsulation and processing ensures compatibility with a broad range of animal and human cell types with high viability of selected clones
- Speed and Efficiency: Reduces Discovery and Cell Line Development process timelines and enables multiple projects to be run in parallel. No downtime between runs
- Sterility: The benchtop system can be utilized in Class II biosafety cabinets with end-to-end sterility and disposable consumables

 Download the full white paper
Download the full white paper
Acknowledgments
Produced from materials originally authored by Fluidic Sciences and Sphere Bio. The work detailed in this article was partially funded by the UK Government Advanced Manufacturing Supply Chain Initiative (AMSCI) under the BioStreamline Project, which exists to develop new approaches for the more efficient development and manufacture of next-generation biologics.
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.