The growing demand for more rapid high-throughput cell-line development and improved screening in the pharmaceutical space is motivating research facilities and companies to automate their labs.
Though robotics has been present in some laboratories for around four decades, it is becoming increasingly common. It helps to free researchers of repetitive tasks by allowing more walk-away time.
Simple to complex assays can be automated to reduce the workload of unskilled steps, such as centrifuging, sample handling, moving to racks, and liquid handling. New trends in robotics facilitate better, more compact, and easy-to-program robots, which could be simple to incorporate into an integrated workcell solution.1,2
Standard cell-line development necessitates screening tens of thousands of clones to identify the best stable cells producing high levels of bioproducts.
The automation of a mammalian cell-line development workflow involves automated, integrated screening of transfected cells for edits, monoclonality, and growth assessment, as shown in Figure 1.
This system contains numerous instruments and a collaborative robot to execute all steps, from dispensing a single cell to screening and expanding monoclonal cells for further validation assays. This integrated system raises the throughput from low to high and removes human error by increasing consistency.
The cells are kept in the greatest possible conditions and monitored for the week, including weekends. This reduces the production timeframe drastically and allows faster scalability.
This automated workcell solution comprises all the instruments detailed in Figure 1. Other instruments are straightforward to add based on the needs or complexity of the assays.3,6
Case study - Automated screening of CRISPR-edited cell lines for monoclonality
The integrated system had a single-cell printer, Clone Select Imager – Florescence (CSI-FL), an automated incubator, hotels, a liquid handler, barcode readers, and a collaborative robot.
The transfected cells were printed into 96 and 384 well plates using the system and subsequently imaged for Day 0 monoclonality assessment and regularly monitored for growth.
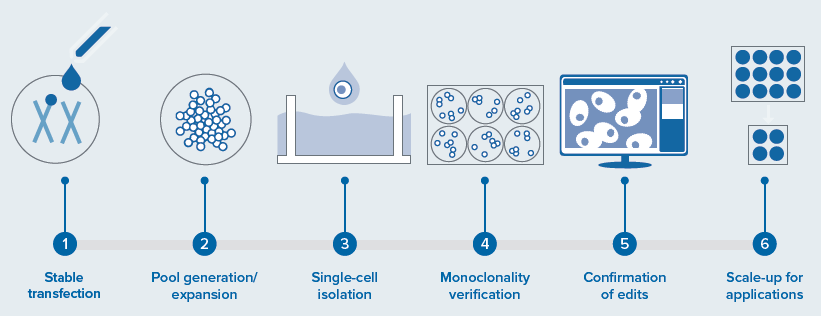
Image Credit: Molecular Devices UK Ltd
The complex workflow involved with these tools increased the throughput and automation of gene editing assays, screened the cells for monoclonality, and ran the endpoint assays. This solution can be applied to cells and the development of CRISPR-edited organoids for various applications (see Figure 1).
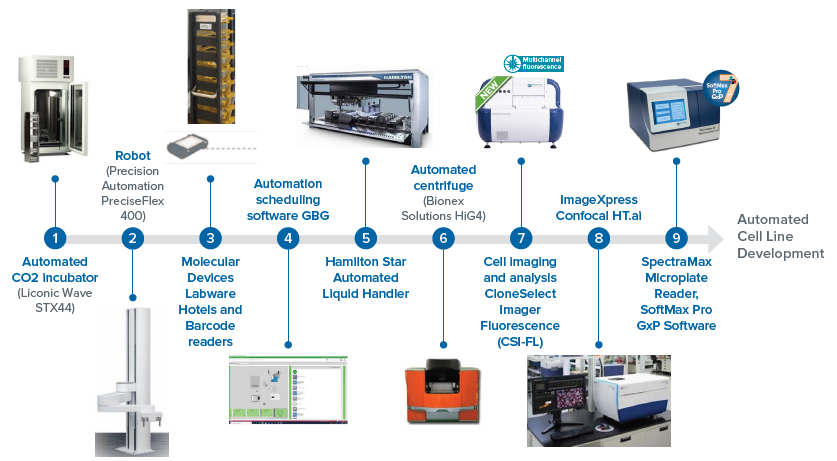
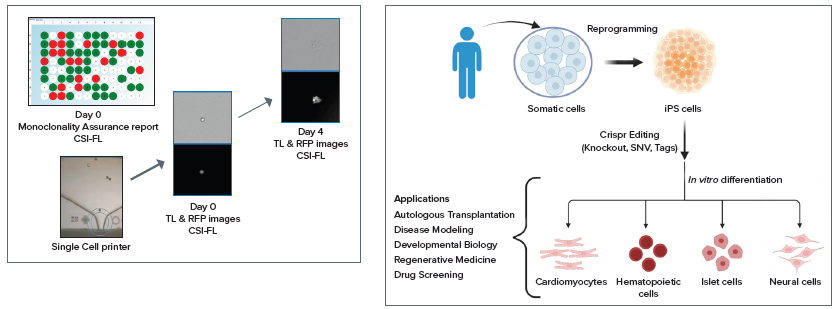
Figure 1. Workflow steps involved in a cell line development and integrated automated system for a CRISPR-edited cells/organoid screening—disease modeling. Includes various instruments and automation of different steps: the screening, and monitoring for cellular monoclonality being the most critical (performed by CSI-FL) and potential applications. Image Credit: Molecular Devices UK Ltd
The instrument operations were scheduled in advance with the utilization of automated laboratory scheduling software (GBG, as shown in Figure 2) to run the workflow steps.
CRISPR editing of the cells and maintenance were conducted using an incubator and a liquid handler. Subsequently, these cells were single-cell dispensed into multi-well plates for monoclonal cell-line development.
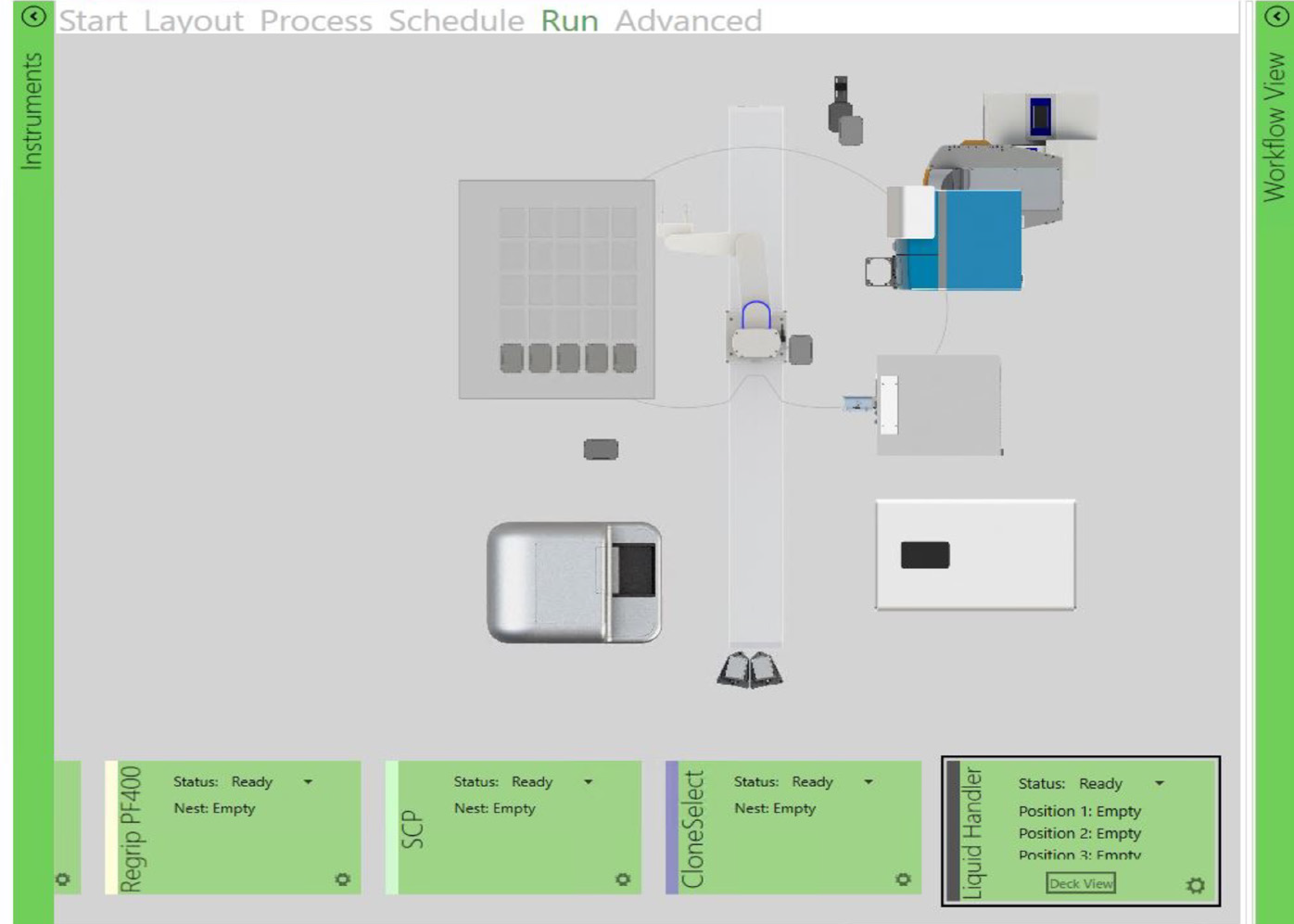
Figure 2. This virtual platform shows CSI-FL, automated incubator, liquid handler, hotel, and barcode readers. These instruments are integrated on a fully virtual environment (GBG) across a workstation to run an automated workflow. The devices were monitored in real-time and added or dropped off of the system based on the workflow needs. Image Credit: Molecular Devices UK Ltd
These multiwell plates were imaged daily, from Day 0 to Day 14, utilizing CSI-FL (as shown in Figure 3 and Figure 4) to produce a day 0 monoclonality assurance report (see Figure 1) and monitor the growth of each cell.
The cells were tracked electronically, and monitoring parameters were stored in plate data: cell number estimation, cell confluence, and growth curve.
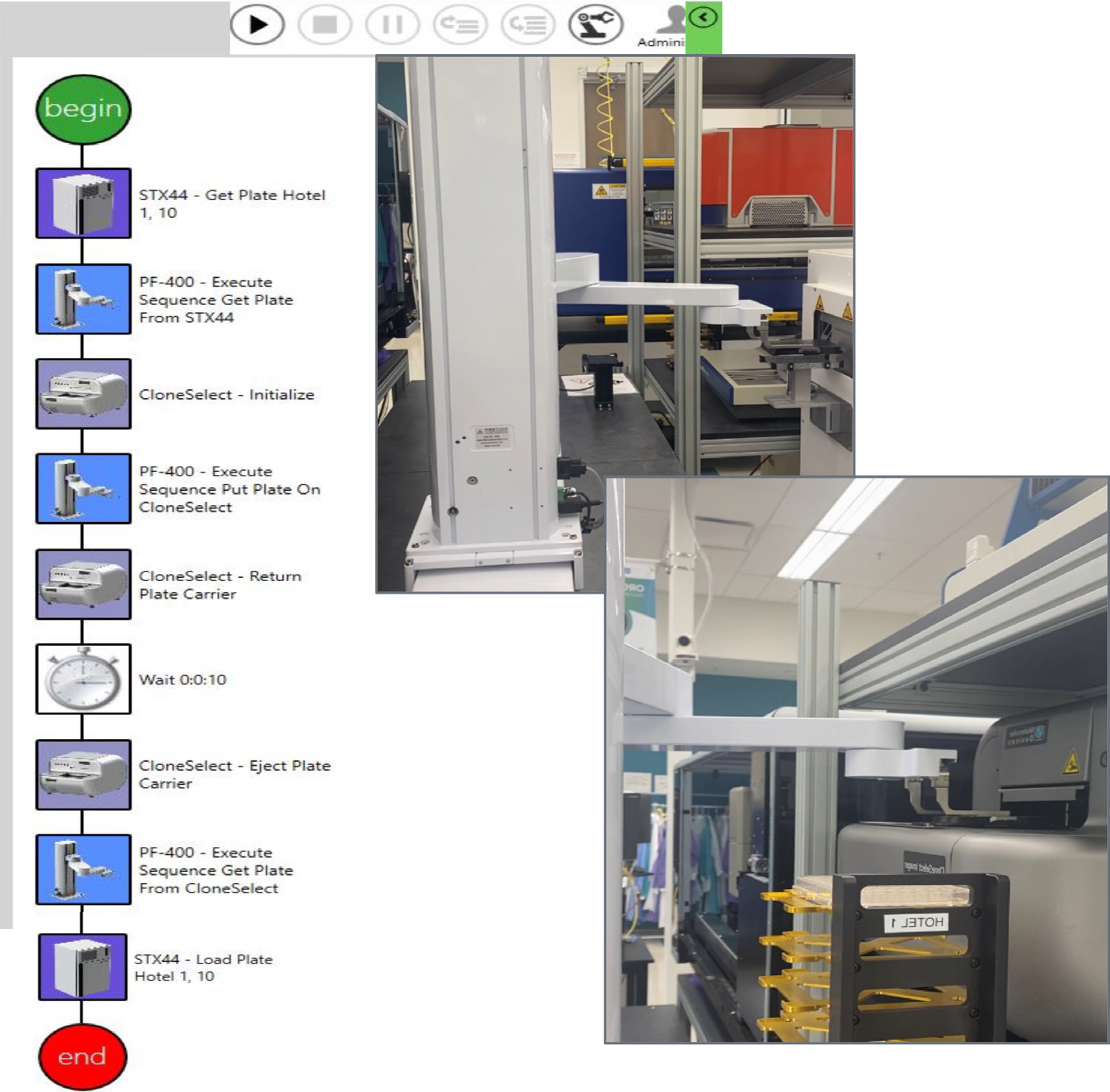
Figure 3. This sequence of steps was designed on automation software (GBG, left) to image a single-cell printed 96-well plate. The path is timed/scheduled and allows imaging – incubator → imager → incubator. The top right image shows the collaborative robot picking up the 96-well plate,and the bottom right image shows the robot placing the plate in CSI-FL. Image Credit: Molecular Devices UK Ltd
The instrument quantitatively and objectively evaluates cell growth with compatibility for adherent or settled suspension cell types, including diverse cell types such as HEK, CHO, hybridomas, IPSCs, and many more.
CRISPR plasmids for p53 KO (Santa Cruz Biotechnology) were used to build an adherent monoclonal HEK-293 cell line via transfection. This cell line was then subjected to endpoint assays for edit verification.7-9
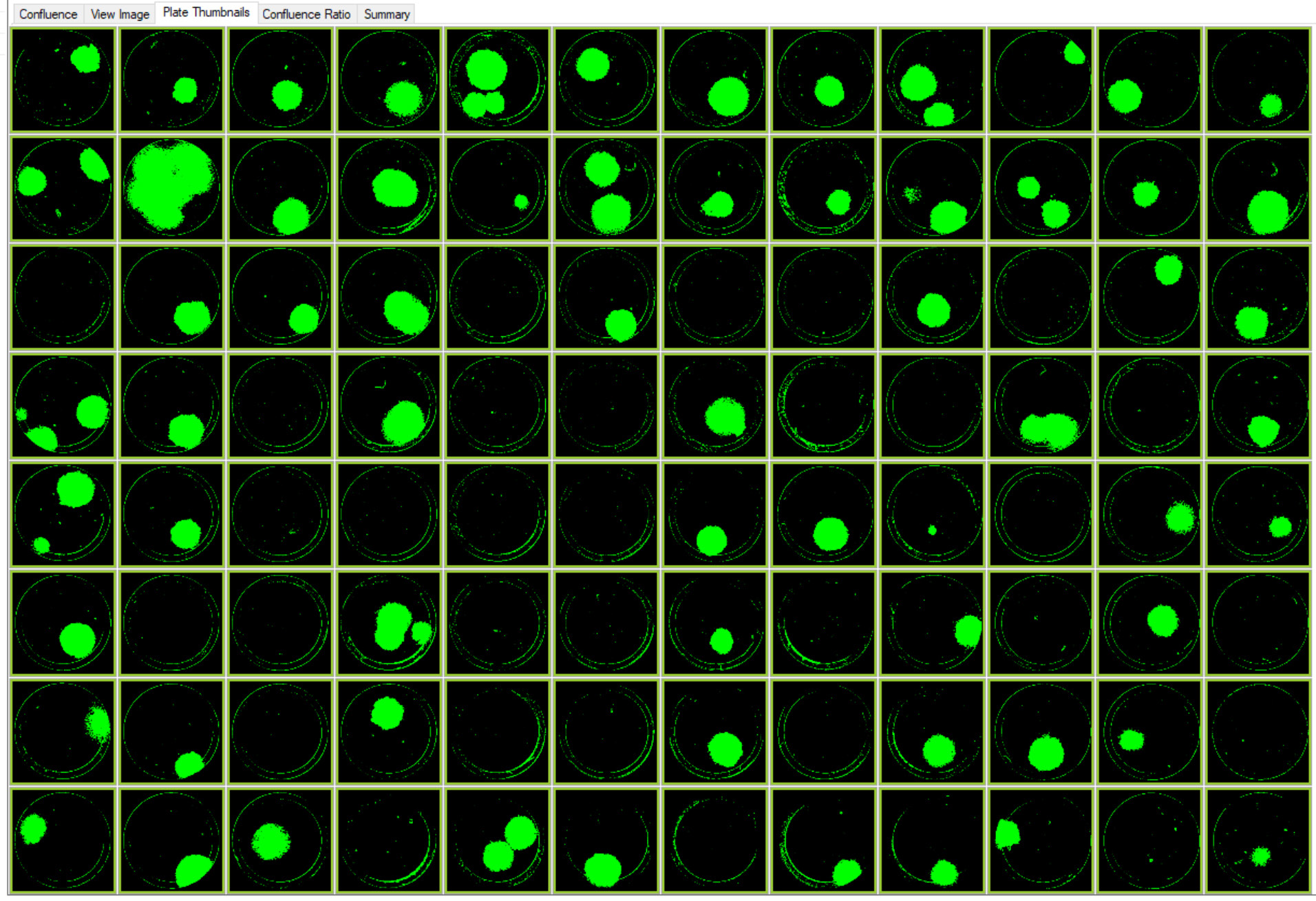
Figure 4. Images of colonies of single-cell printed CRISPR edited cells with RFP marker. This plate layout image of a 96–well Costar 3300 plate with cells on Day 9 was acquired using CSI-FL at 4X. Image Credit: Molecular Devices UK Ltd
Summary
With CSI-FL and automated instruments, the edited cells were easily imaged and tracked for monoclonality with weeks of walk-away time. The reliability, throughput, and chances of contamination were significantly changed. High-quality CRISPR-edited cell lines were acquired and utilized for end-point assays.
In response to the ever-growing demand to reduce timelines for cell-line development and automated solutions, CSI-FL paired with automated instruments offers an automated integrated systems workflow.
Benefits include quick scale-up acceleration, consistent processing, increased walk-away time, reduced redundant tasks, better cellular monitoring, and dynamic workflow solutions.
References and further reading
- Lindgren, Kristina, et al. “Automation of cell line development.” Cytotechnology 59.1 (2009): 1-10.
- F. Mirasol, “The Role of Automation in Cell-Line Development,” BioPharm International 32 (1) 2019.
- Felder, RA, Boyd, JC, Savory, J, Margrey, K, Martinez, A, Vaughn, D. Robotics in the clinical laboratory. Rev Clin Lab Med 1988; 8:699–711. https://doi.org/10.1016/s0272-2712(18)30657-7.Search in Google Scholar
- Wheeler, MJ. Overview on robotics in the laboratory. Ann Clin Biochem 2007; 44:209–18. https://doi.org/10.1258/000456307780480873.Search in Google Scholar
- Lippi, G, Da Rin, G. Advantages, and limitations of total laboratory automation: a personal overview. Clin Chem Lab Med 2019; 57:802–11. https://doi.org/10.1515/cclm-2018-1323.Search in Google Scholar
- Chapman, T. Lab automation and robotics: Automation on the move. Nature 421, 661–663 (2003). https://doi.org/10.1038/421661a
- Accelerating gene edited cell lines with the CloneSelect Imager FL, Molecular Devices.
- CloneSelect Imager FL fluorescent imaging for rapid day zero monoclonality assurance, Molecular Devices.
- What is Gene Editing, CRISPR Engineering, CRISPR/Cas9 | Molecular Devices
About Molecular Devices UK Ltd
Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.