Most potential oncology drugs fail during the drug development pipeline, even when there has been promising data for their efficacy during the in vitro stage. This makes it vital to identify in vitro models that are better at recapitulating tumor biology.
While two-dimensional (2D) cell culturing remains the main method employed in drug screening, it is less physiologically relevant than three-dimensional (3D) culturing.
Three-dimensional cell modeling has issues around assay reproducibility, scalability, and cost, which have been a barrier to its widespread adoption as a primary screening method in the drug discovery sector.
Biological readouts from 3D models are generally limited to one or a small number of features that do not fully capture the complex biological nature of tumoroids.
Image-based phenotypic profiling, such as the Cell Painting assay, is increasingly common in various applications to quantitatively capture a wide range of phenotypic changes in response to compound-induced or genetic perturbations.
In this study, a screening was carried out utilizing patient-derived 3D spheroids (tumoroids). In addition to readouts on cell viability, the Cell Painting assay was adapted for the 3D tumor model.
These tumoroids were formed from primary cells isolated from a patient-derived tumor explant, TU-BcX-4IC. The explant represents a triple-negative breast cancer subtype of metaplastic breast cancer.
Tumoroids were treated with 168 compounds from the NIH library of approved oncology drugs. Cell Painting was employed to facilitate the evaluation of associated phenotypic changes.
A single-feature readout from an image-based viability assay was also performed in parallel for comparison purposes.
A total of 24 hits were identified based on the phenotypic distance score. This score was calculated via the principal component analysis (PCA).
Two-thirds of the hits were found to overlap with hits from the image-based viability assay. These results demonstrated that Cell Painting represents a feasible additional and important 3D cell model analysis approach.
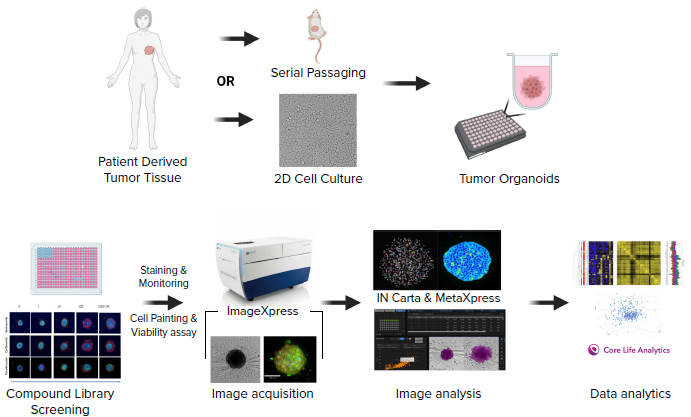
Schematic diagram of the experimental workflow including generation of a patient-derived cell line, formation of tumoroids in 384 well U-shape, low attachment plates, compound treatment, staining, imaging, and analysis. Image Credit: Molecular Devices UK Ltd
Methods
Cell culture
Methods for generating tumoroids and PDX organoids (PDXO) are well established (Matossian, et al., 2021). In this instance, the primary tumor sample was implanted into SCID/Beige mice. It displayed rapid tumor growth and, within 14 days, had reached maximal tumor volume >1000 mm3.
A cell line was then generated from that sample and was expanded in 2D culture and tumoroids were formed from 4IC cells expanded in 2D. The 4IC cells were dispensed ~2,000 cells per well (in U-shape low attachment 384 plates [Corning]) and subsequently incubated for 48 hours until they formed tight tumoroids.
The resulting 4IC cells were cultured with Advanced DMEM supplemented with NEAA, 2 mM glutamine, glucose, and insulin 120 μg/L, 10% FBS (Gibco 12491-015). In the case of metabolic assays, the tumoroids were cultured with DMEM + 10% dialyzed serum (2 mM glutamine, 5 mM glucose, without phenol red).
Spheroid monitoring and imaging
The fluorescent images' transmitted light (TL) was acquired on the ImageXpress® Confocal HT.ai High-Content Imaging System (Molecular Devices) utilizing MetaXpress® High-Content Image Analysis Software.
Tumoroid images were obtained in TL with an approximately 60 µm offset, while Z-stack images were obtained with the 10x or 20x objectives utilizing confocal mode. MetaXpress or IN Carta® Image Analysis Software was employed in the analysis.
Cell Painting assay and data analysis
For the Cell Painting assay 17–20, the 3D TU-BcX-4IC tumoroids were labeled utilizing a protocol modified from Bray et al. The tumoroids were incubated with MitoTrackerDeepRed (500 nM) (cat #) for two hours.
The samples were fixed with 4 % PFA in HBSS for one hour. Each wash step was carried out by exchanging half the volume in each well with HBSS. This was carried out to minimize the displacement of tumoroids from the well’s center.
Following fixation, the samples were subsequently washed three times with HBSS. For permeabilization, samples were incubated with 0.1 % Triton X-100 (in HBSS) for two hours at room temperature and washed with HBSS.
The dyes were prepared in HBSS and 1 % BSA (wt/vol) and then incubated overnight with the following final concentration: Phalloidin750 (15 μl/ml), Hoechst (15 μg/ml), ConcanavalinA-488 (250 μg/ml), Syto14 (7.5 μM), WGA (3.75 μg/ml).
Results
Spheroid culture and imaging
The 3D cancer culture was started from primary triple-negative tumor. The cell line was developed by passaging primary tissues in SCID mice, then adapting these for 2D cell culture.
The tumoroids were formed via the culturing of 2,000 cells in 384 well low-attachment plates for 48 hours. The tumoroids were then treated with compounds from the National Cancer Institute (NCI) library of approved anti-cancer drugs. Five concentrations were used for testing (Figure 1).
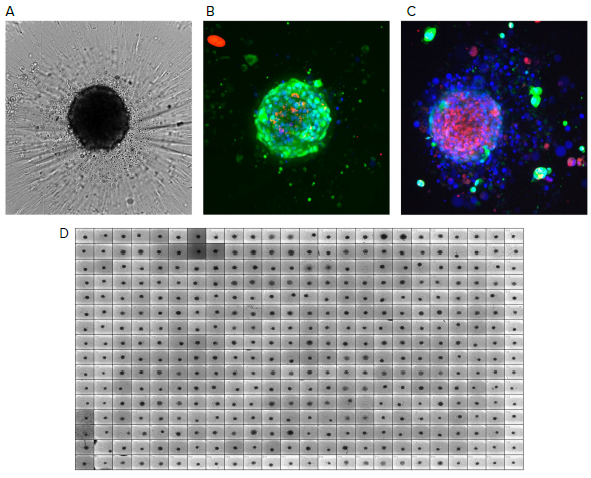
Figure 1. Reference images of tumoroids. A) Tumoroids formed 48h after plating, TL images (10X); B) Composite fluorescent images of untreated tumoroid stained with calcein AM (green), EthD-1 (red) and Hoechst (blue); C) Tumoroids treated with romidepsin (10 nM). Tumoroids were imaged using confocal option of the automated imaging system, Z-stack of 15 images was taken 10 μm apart, then maximum projection images were created (shown). D) 384 well plate with tumoroids after compound treatment, TL images. Image Credit: Molecular Devices UK Ltd
High-content imaging and analysis of cancer spheroids
Tumoroids were treated with compounds at five different concentrations (Figure 2) and subsequently screened based on cell viability. Phenotypic readouts that included total cell count, tumoroid area, and fluorescent intensity were also quantified.
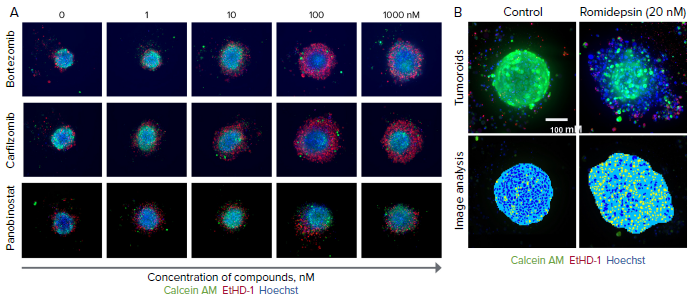
Figure 2. Tumoroids were treated with compounds for 5 days then stained with calcein AM (green), EthD-1 (red), and Hoechst (blue). A) Selected confocal images, 10X shown. Note dose-dependent dis-integration of tumoroids, increase in cell death indicated as increase of EthD-1 staining (in red). B) End-point analysis of fluorescent images was done using Custom Module Editor in MetaXpress. Images of the nuclei of treated and untreated tumoroid shown. Analysis masks show tumoroids projection in blue and nuclei in yellow. Tumoroid area and count nuclei were used as main read-outs for phenotypic characterization of tumoroids and compound effects. Image Credit: Molecular Devices UK Ltd
Deep learning-based segmentation for label-free image analysis
Utilizing IN Carta software allows for adjustments in the image analysis routine to achieve robust detection of objects of interest (Figure 3). A deep-learning semantic segmentation module (SINAP) may be employed to improve the detection of challenging features.
A new model was developed to segment images of cancer spheroids acquired in transmitted light. The spheroid segmentation mask then derived over 200 features (from all seven imaging channels).
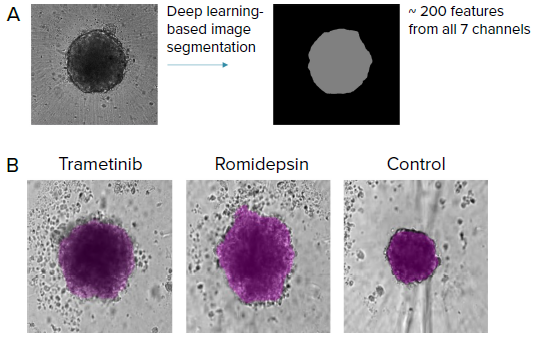
Figure 3. Automated image analysis of tumoroids was done using transmitted light images (label-free) (10X) with AI-based image analysis IN Carta software. A) SINAP (IN Carta) was used to develop a model to segment tumoroids. Over 200 features were then extracted from the segmentation mask from all 7 imaging channels (cell painting) that include stains for mitochondria, nuclei, ER, golgi, actin, RNAP and nucleoli. B) Examples of label-free images from tumoroids treated with different compounds and the corresponding segmentation mask (purple) from SINAP shown. Note that the SINAP model can identify tumoroids that are phenotypically different. Image Credit: Molecular Devices UK Ltd
Results
Cell painting in phenotypic analysis of 3D spheroids
To allow for a more in-depth investigation into cytotoxic mechanisms elicited by the compounds assayed, other methods of analysis were carried out in parallel to fully characterize phenotypic changes that had been detected. The Cell Painting assay was adapted for 3D tumoroids to evaluate compound effects on tumor phenotype (Figure 4).
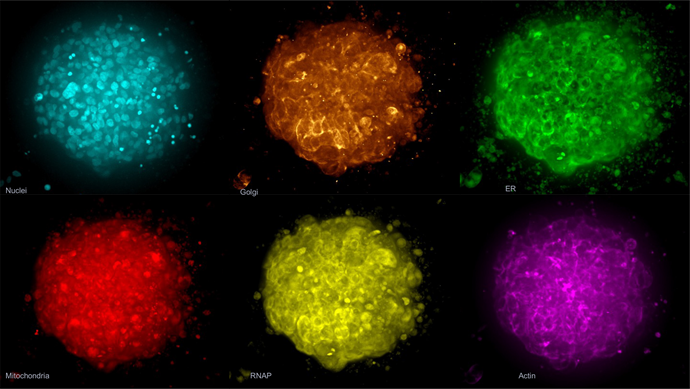
Figure 4. The Cell Painting assay modified for 3D spheroids. Spheroids were labeled with phalloidin, MitoTracker, WGA, SYTO 14, concanavalin A and Hoechst 33342. Shown here is an example image of a control spheroid (maximum projection). Image Credit: Molecular Devices UK Ltd
Data analysis workflow
Measurements obtained via the IN Carta software were subsequently uploaded into HC StratoMineR for further data analysis. StratoMineR is a web-based platform that can guide users through a typical workflow when analyzing high-content multi-parametric data (Figure 5).
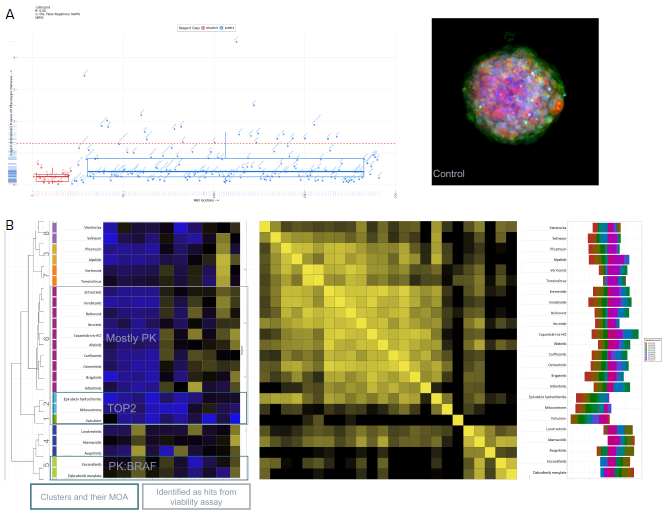
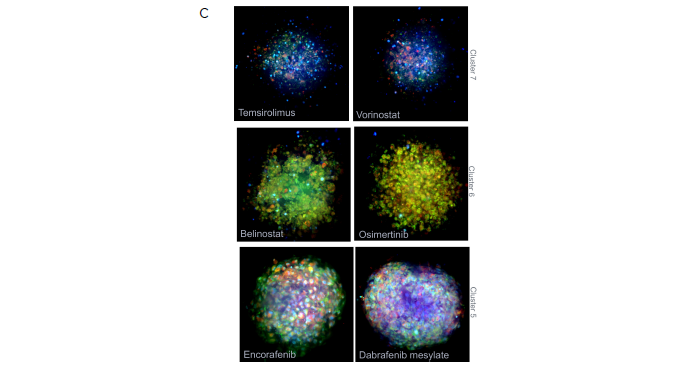
Figure 5. Using StratoMineR for data analysis. A) Scatterplot representing the phenotypic distance score (-log, Y-axis) for each compound is shown. Compound treated samples (blue) compared to negative controls (red) are shown. Hits are identified as those above the red dotted line (p≤ 0.05). B) Results from the cluster analysis are represented as a hierarchical dendrogram. Left: The cluster ID and colored bars indicate which cluster a compound treated spheroid belongs to. Rows represent the included factors and columns represent the compound treatment. Middle: Correlation matrix is shown to give an overview of the similarity/ dissimilarity between the compounds. Columns and rows represent compounds used. The intensity of the color represents the similarity based on the calculated cosine vector score from the PCA factors. Right: Bar graph showing the contribution of each PCA factor to each compound hit. C) Example images grouped by cluster from the Cell Painting assay. Three of the stains, Hoechst (blue, nuclei), SYTO 14 (green, RNAP) and MitoTracker (red, mitochondria) are represented as a composite image. An example of a control DMSOtreated spheroid and spheroids from cluster 7, 6 and 5 are shown. Image Credit: Molecular Devices UK Ltd
Conclusion
The results demonstrated the feasibility of utilizing the Cell Painting assay on 3D cell models such as patient-derived tumoroids.
Phenotypic profiling at the spheroid level was sufficient to identify compounds with cytotoxic and non-cytotoxic effects.
Acknowledgments
Produced from materials originally authored by Angeline Lim, Prathyushakrishna Macha, and Oksana Sirenko from Molecular Devices LLC; Evan F Cromwell from Protein Fluidics, Co; Margarite D Matossian, Courtney K Brock, and Matthew E Burow from Tulane University; and Victor Wong, David Egan, and Wienand Omta from CoreLife Analytics.
About Molecular Devices UK Ltd

Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.