Microalgae are photosynthetic organisms that grow in water systems. They convert atmospheric CO2 into organic molecules like lipids, carbohydrates, and other bioactive compounds.
These biological properties mean that microalgae present a renewable and sustainable source of food ingredients. These include animal-free omega-3, alternative proteins, and natural pigments utilized in the food and beverage industry.
Microalgae’s potential to meet dietary requirements has attracted the attention of the food industry. The European Food Safety Authority (EFSA) has stated that the demand for natural food pigments is growing among consumers who favor natural products over possibly toxic synthetic food additives.1
However, natural pigments may be vulnerable to light exposure and can change color when exposed to certain environmental conditions, such as temperature and pH. They are also typically only available in a limited range of colors. All the above factors motivate the R&D surrounding the use of new natural pigments as food dyes.
Discovering microalgal pigments with valuable properties for the food industry demands high throughput automation to screen many microalgal strains quickly.
Using this technology, Fermentalg® labs have leveraged Galdieria sulphuraria metabolism to create a natural blue pigment rich in C-phycocyanin. This is extracted and commercialized as BLUE ORIGINS®.2
BLUE ORIGINS® is a natural pigment with beneficial properties, including stability at high temperatures and low pH compared to other microalgae-derived dyes. It is well-suited for various food and beverage industry applications, including beverages, candies, and ready-to-eat cereals.
C-phycocyanin is currently the only blue pigment in the FDA food coloring agent-exempt product list, meaning it is suitable for the manufacture of natural products.3
Fermentalg® scientists have created innovative techniques for screening and selecting microalgal strains with the QPix 420 microbial colony picker (from Molecular Devices®).
Conventionally, colony picking is conducted manually using sterile pipette tips or inoculation loops. This makes it typically a slow and labor-intensive process.
The QPix 420 system facilitates high throughput colony picking, with over 3,000 colonies picked in one hour and over 98% picking efficiency.
A range of fluorescent filter sets provide increased system flexibility and walk-up operations, which allows the phenotypic selection of unique colonies based on morphological features and levels of fluorescent pigments or fluorescent protein expression.
Benefits of fluorescence on QPix® 420
- Quantitative fluorescent screening enables efficient and objective selection of unique clones
- Maintain subsequent clonal integrity during bio-production
- Experimental flexibility is increased with a variety of fluorescent filter sets
- Simple software ensures the correct colony is picked based on the defined experimental parameters
Materials and methods
While searching for a strain of Galdieria sulphuraria that generates high levels of C-phycocyanin, Fermentalg® acquired and preserved a set of variants with varying C-phycocyanin contents.
These strains were plated on Gross-agar medium (with a pH of 3) and underwent incubation for two weeks at 37 °C with 60% humidity in the dark.4
The imaging and analysis of all plates took place using the QPix 420 system, which facilitates fast walk-up operations. The utilization of a suitable pin type guarantees compatibility with different microorganisms. In this case, Christmas tree-shaped pins were employed to pick and inoculate G. sulphuraria colonies.
Sterility was preserved using three wash baths to sanitize the pins, as shown in Figure 1.
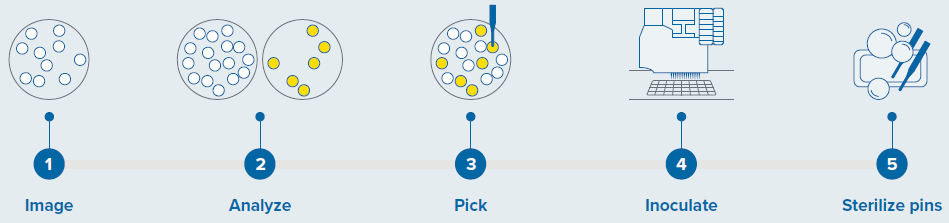
Figure 1. Colony screening workflow with QPix 420 system. Image Credit: Molecular Devices UK Ltd
Results
A fluorescent approach to screen G. sulphuraria colonies
A fluorescence-based method was utilized to select colonies of the microalgae Galdieria sulphuraria. This organism is rich in C-phycocyanin, a water-soluble protein found in blue-green algae. This protein is responsible for the characteristic bright blue color of the algae.
C-phycocyanin emits fluorescence with a peak at 642 nm when excited with a red light.5
Two-week-old colonies of G. sulphuraria were imaged and screened under transmitted light and fluorescence using the QPix 420 system, which allows the objective identification and selection of colonies that generate the fluorescent pigment of interest (C-phycocyanin in this case).
Initially, a test image of the plate under transmitted light and the selected fluorescence is obtained using the QPix Fusion Software (as shown in Figure 2A and B, respectively). The test images may be modified by defining the acquisition settings, such as exposure time.
The detected colonies are visualized using a green overlay created by the software algorithm in correspondence with each detected feature.
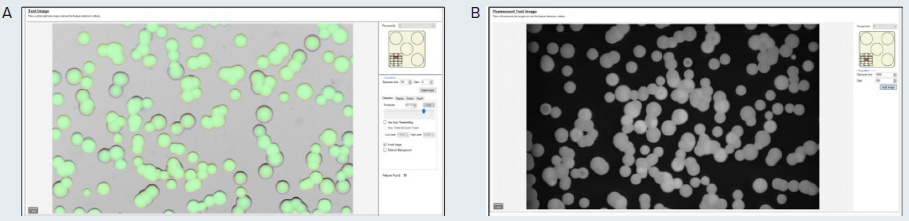
Figure 2. (A) Test image in transmitted light of the microalgae G. sulphuraria. QPix Fusion Software™ shows detected features creating a green overlay on each colony. (B) Test image of the same sample was acquired in fluorescence channel (Ex/Em filter: 628/692nm) to detect colonies expressing C-phycocyanin. Image Credit: Molecular Devices UK Ltd
Once the images are obtained, the QPix Fusion Software analyses them based on the feature properties. The software identifies the location of separate colonies on the culture plate (Petri plate, QTray, or Omnitray).
The flexibility of the Fusion software enables the user to define the desired colonies based on morphology, including compactness, colony size, diameter, axis ratio, and minimum proximity.
Selected colonies of G. sulphuraria were either included (as shown in Figure 3 in yellow) or excluded (as shown in Figure 3 in orange) based on the selection criteria.
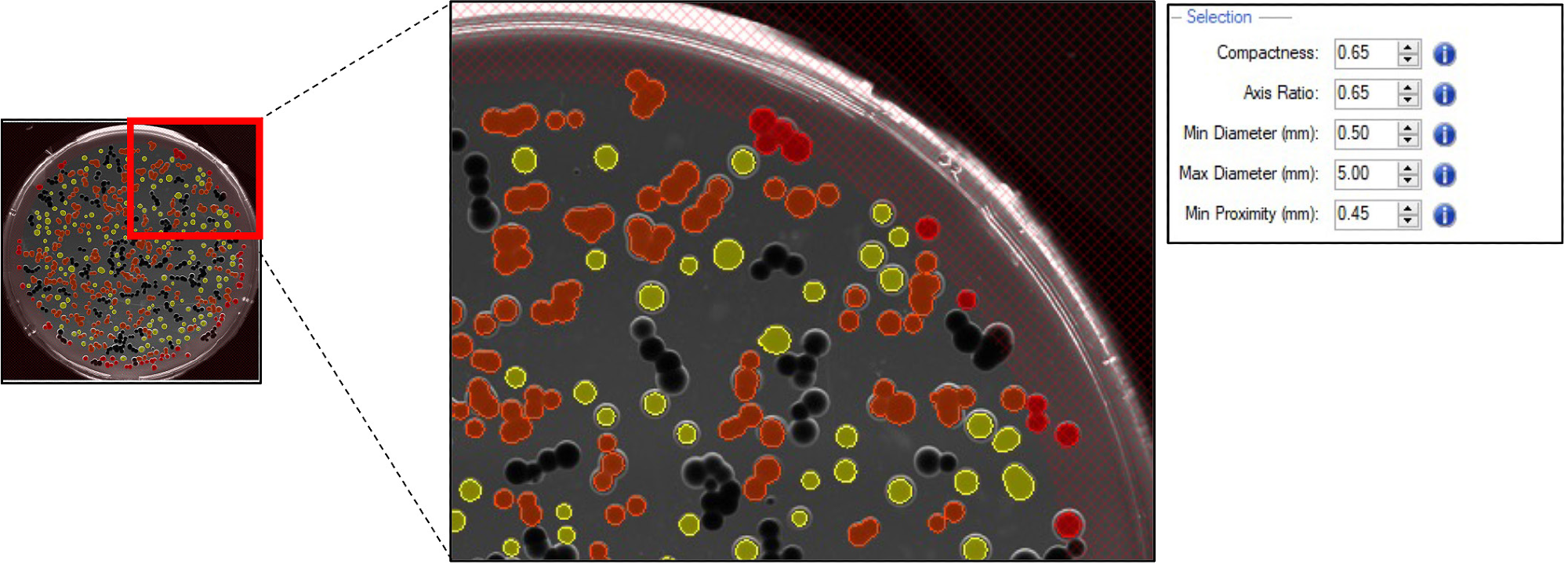
Figure 3. Pickable colonies are displayed in yellow. Orange: colonies excluded according to selection criteria. Top right panel: selection criteria defined for accurate colony picking. Image Credit: Molecular Devices UK Ltd
The quantitative and tunable fluorescence intensity threshold determines the desirable protein expression level. The range of fluorescence must be adjusted to select cells that contain the highest concentrations of C-phycocyanin.
For this experiment, colonies that generate C-phycocyanin with a Mean Fluorescence Intensity (MFI) value of 36,000 to 43,000 were selected (as displayed in Figure 4A and B, respectively).
Once selection based on the user-defined criteria occurred, the colonies were accurately picked using the QPix 420 system.
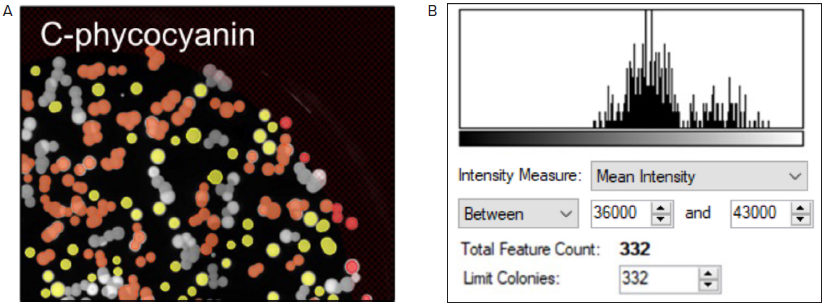
Figure 4. Fluorescent screening of G. sulphuraria colonies producing C-phycocyanin. (A) Representative fluorescent image of G.sulphuraria colonies expressing different levels of C-phycocyanin. The Red filter pair (Ex/Em: 628/692) was used. (B) Fluorescence histogram displayed as Mean Intensity. Image Credit: Molecular Devices UK Ltd
Screening and detection of high-expressing C-phycocyanin colonies
Distinct G. sulphuraria strains that generate varying concentrations of C-phycocyanin were analyzed with the QPix 420 system to test the capability of the system to identify and select the high-expressing C-phycocyanin colonies, which appear as dark green (as displayed in Figure 5 A).
The results were as expected, with colonies with the lowest concentration of C-phycocyanin appearing white on agar plates, and the colonies that expressed average pigment levels appearing light green.
The different strains were then imaged in transmitted light using the QPix 420 and subsequently screened based on C-phycocyanin fluorescent emission (as shown in Figure 5B-C).
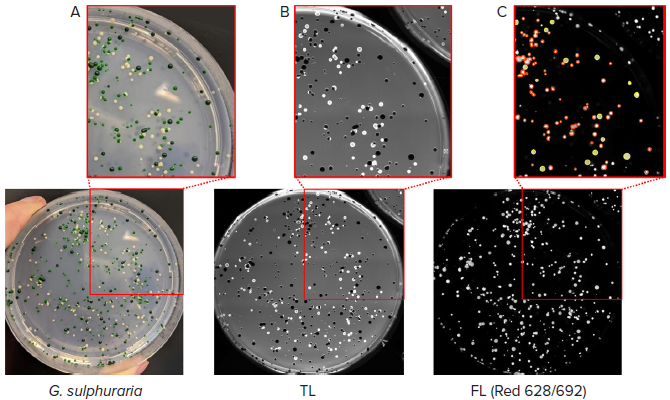
Figure 5. Selection of high-expressing C-phycocyanin colonies. (A) Colonies of G. sulphuraria with different pigmentation were analyzed. (B) Transmitted light (TL) image of the same plate of colonies detected with QPix 420 camera. (C) Fluorescent image (Red filter pair, Ex/Em: 628/692nm) of G. sulphuraria colonies. Pickable colonies are displayed in yellow. Orange: colonies excluded according to selection criteria. Image Credit: Molecular Devices UK Ltd
A fluorescent signal for the dark green- and light-green-colored colonies was detected. The successful selection and picking of the dark green colonies were carried out based on the previously tested Mean Fluorescence Intensity values ranging from 36,000 to 43,000.
The disappearance of the white colonies in fluorescent imaging proved the absence of C-phycocyanin in this population of microalgae.
Conclusion
The interest in microalgae within the food industry is growing due to the increased demand for sustainable and natural food ingredients.
Fermentalg® is utilizing microalgae metabolism for the development and screening of multiple G. sulphuraria strains generating a high temperature- and low pH-stable natural blue pigment as a food ingredient (BLUE ORIGINS®).
To increase the speed of this product reaching the industry, the automated colony screening of G. sulphuraria colonies was conducted.
High throughput colony picking provides substantial benefits over traditional screening techniques, such as more rapid identification of desired clones which streamlines the subsequent downstream analysis.
The fluorescence capabilities of the QPix 420 were employed for the selection of clones that produce the highest concentrations of C-phycocyanin, accelerating the scale-up of microalgae-based natural pigment production.
In general, high throughput automation shows great promise for the future development of microalgal products.
Acknowledgments
Produced from materials originally authored by Carola Mancini from Molecular Devices, Audrey S. Commault, Rodrigo Rangel, and Julien Demol from Fermentalg.
References and further reading
- Carocho M, et al. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr Rev Food Sci Food Saf. 2014 Jul;13(4):377-399. doi: 10.1111/1541-4337.12065. PMID: 33412697
- Athane et al., The safety evaluation of phycocyaninenriched Galdieria sulphuraria extract using 90-day toxicity study in rats and in vitro genotoxicity studies. Toxicology Research and Application 2020.
- Minxi Wan et al., Comparison of C-phycocyanin from extremophilic Galdieria sulphuraria and Spirulina platensis on stability and antioxidant capacity, Algal Research 2021, Volume 58.
- Gross, W. & Schnarrenberger, C. (1995): Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol. 36(4): 633-638.
- Niels T. Eriksen, Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Applied Microbiology and Biotechnology, 2008
Molecular Devices UK Ltd
Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.