The utilization of 3D organoids is increasingly prevalent in disease modeling and drug discovery due to their ability to better represent biologically relevant tissue architecture, microenvironments, and functionality. However, the complex nature of 3D models is a potential obstacle to their greater utilization in drug screening and research.
Molecular Devices LLC aims to overcome these obstacles by applying automation to labor-intensive manual protocols. As a result, the company has established automation protocols for the entire 3D culture workflow process and increased organoid assays' accuracy and throughput.
These automated methods employ an integrated work cell, facilitating automated cell plating, media exchange, culture monitoring, and high-content imaging. They also facilitate the automation of cell staining, endpoint assays, and compound addition.
As a result, automation can be employed in the two most widely utilized organoid workflows. These are:
- Organoids cultured in matrix (for example, Matrigel) that includes primary iPSC- or tissue-derived colorectal or intestinal organoids
- Organoids that have been cultured matrix-free by utilizing U shape ULA labware, employed in spheroids and patient-derived breast cancer tumoroids
Process and benefits of automation
Integrating several instruments automated the workflow for organoid assays. This facilitated the automated culturing of organoids, compound treatment, maintenance, and end-point assays for 3D cellular models. This method facilitates the automation and scaling up of complex assays in 3D biology, and can also be employed in disease modeling and compound screening.
Materials and methods
Cell culture
The processes for generating PDX organoids (PDXO) and tumoroids have been previously established (Matossian, et al 2021). The primary tumor sample was implanted into SCID/Beige mice and exhibited rapid tumor growth, taking 14 days to reach maximal tumor volume >1000 mm3.
A cell line was subsequently generated from that sample which was capable of being expanded in 2D culture. Tumoroids were formed from 4IC cells which had been expanded in 2D and 4IC cells were dispensed ~2,000 cells per well (in U-shape low attachment 384 plates, Corning) and then incubated for 48 hours until they had formed tight tumoroids.
These 4IC cells were cultured with Advanced DMEM supplemented with 2 mM glutamine, NEAA, glucose, and insulin 120 µg/L, 10 % FBS (Gibco 12491-015).
In the case of metabolic assays, the tumoroids were cultured with DMEM + 10 % dialyzed serum (2 mM glutamine, 5 mM glucose, without phenol red).
The 3D intestinal organoids were derived from primary mouse intestinal cells or human iPSCs (STEMCELL Technologies). These cells were then cultured and differentiated in accordance with STEMCELL Technologies’ protocol.
IntestiCult™ Organoid Growth Medium (STEMCELL Technologies) was utilized for cell culture. The cells were seeded in 50 % growth-factor reduced Matrigel (Corning) domes in a 24-well plate format. These were fed fresh media every second day for 7 to 10 days. Subsequently, intestinal organoids were passaged-dissociated and re-plated into fresh Matrigel domes.
It is important to note that this project was carried out under the license from HuB Institute.
Cell monitoring and imaging
Employing MetaXpress High-Content Image Analysis Software allowed for transmitted light (TL) of fluorescent images to be obtained utilizing the ImageXpress Confocal HT.ai High-Content Imaging System (Molecular Devices).
Tumoroid images were attained in TL with an offset of approximately 60 µm, while Z-stack images were obtained with the 10X or 20X objectives by employing the instrument’s confocal mode. IN Carta™ or MetaXpress Image Analysis Software were utilized for analysis.
Automation of cell culture and imaging protocols
Automated imaging and analysis of organoids are vital for quantitatively assessing phenotypic changes in organoids and increasing test and experiment throughput.
The construction of an automated, integrated system enabled the automation of monitoring and maintenance, the characterization of growth and differentiation of organoids and stem cells, and testing of the effects of a range of compounds.
Features of the automated system included the ImageXpress Confocal HT.ai system and analysis software, Biomek i7 or Hamilton liquid handler, automated CO2 incubator, and collaborative robot and rail. The robotic automation was enabled by utilizing the Green Button Go Scheduler.

Figure 1. The integrated system includes an automated CO2 incubator, liquid handler, IXM-C HT.ai confocal imaging system, automation compatible plate reader and centrifuge A Collaborative robot and scheduling software allows automated plate transfer between instruments, and scheduling of different steps. Image Credit: Molecular Devices UK Ltd
Results
Automated culturing 3D organoids in Matrigel
Culturing 3D organoids in Matrigel or other matrices involved a series of processes:
- The seeding primary or iPSC-derived cells mixed with Martigel into the “domes”
- 8 to 10 days of culture with media exchange every second day. Growth and maturation of organoids was monitored via automated imaging in transmitted light.
- Organoids were harvested after re-suspension of domes and were dis-integrated by utilizing either enzymatic treatment or mechanical repeated pipetting.
- Cell pellets purified by centrifugation were mixed with Matrigel and subsequently re-plated into new plates.
- An automated liquid handling system facilitated the automated seeding of colorectal, intestinal, or other cells in Matrigel droplets into plates of various formats.
- Automated media exchanges, passaging, and compound addition were then performed.
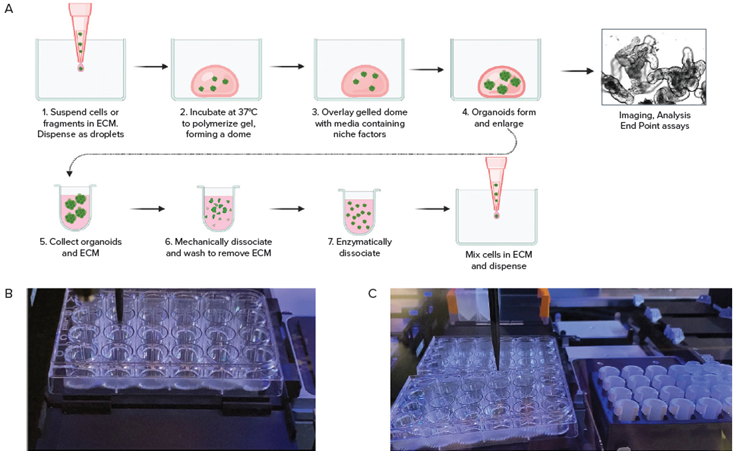
Figure 2. A. Schematic diagram of organoid culture workflow. B. Automated seeding organoids in domes; C. Cell collection by Hamilton liquid handling system. Image Credit: Molecular Devices UK Ltd
Monitoring development of 3D organoids and high content imaging assays
The developing organoids were monitored by employing automated imaging in transmitted light. InCarta SW was used to first find the organoids and then define their size and complexity.
Organoids grown in Matrigel self-organize and develop complex structures, crypts, and lumens. Machine learning-based image analysis facilitated the measurement of organoid size, density, and diameter and characterized their complexity by counting lumens or crypts.
For endpoint measurements, organoids were stained for viability markers or differentiation. Automating confocal imaging and image analysis facilitated the complex phenotypic evaluation of organoid structures in 3D.
Organoid phenotypes were characterized by number, size distribution, cell viability, cell content per organoid, organoid complexity (number of cavities or crypts), as well as mitochondria, nuclei, and cytoskeletons.
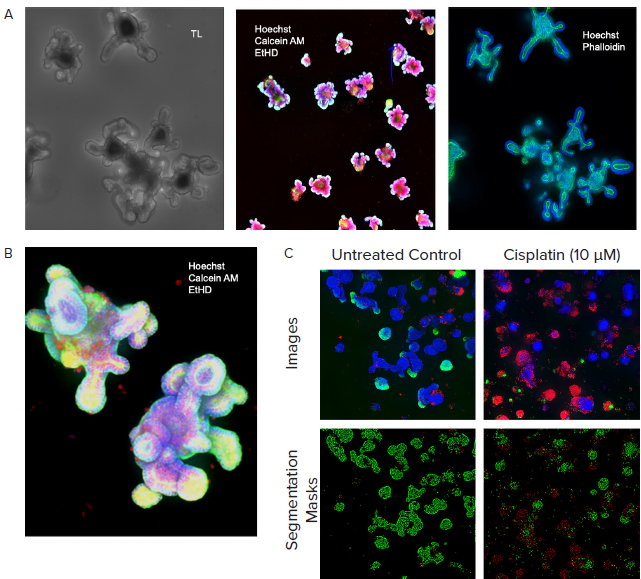
Figure 3. A. Monitored organoid development in transmitted light; live staining of organoids with viability dyes; fixed organoids stained with phalloidin. B. Organoids cultured for 10 days, stained with viability dyes. C. Control and treated organoids stained with viability dyes, live-dead analysis provided by MetaXpress analysis software. Image Credit: Molecular Devices UK Ltd
Culturing and imaging 3D tumoroids
The matrix-free culturing of 3D spheroids or tumoroids usually comprises steps that allow spheroids to form in U-shape low attachment plates. By automating compound addition and liquid exchange, cultures of non-attached microtissues can be handled without losing or breaking those during processing.
The 3D tumoroid culture was started from primary triple negative tumor, while the cell line was developed by passaging primary tissues in SCID mice and then adapting these for 2D cell culture.
Tumoroids were formed by culturing 2,000 cells in 384 well-low attachment plates for 48 hours. These tumoroids were treated with compounds from the NCI (National Cancer Institute) library of approved anti-cancer drugs.
Five concentrations were used for testing, and Biomek automation was utilized in compound dilutions, cell treatments, and staining. Tumoroids were subsequently cultured and monitored daily via the employment of automated imaging.
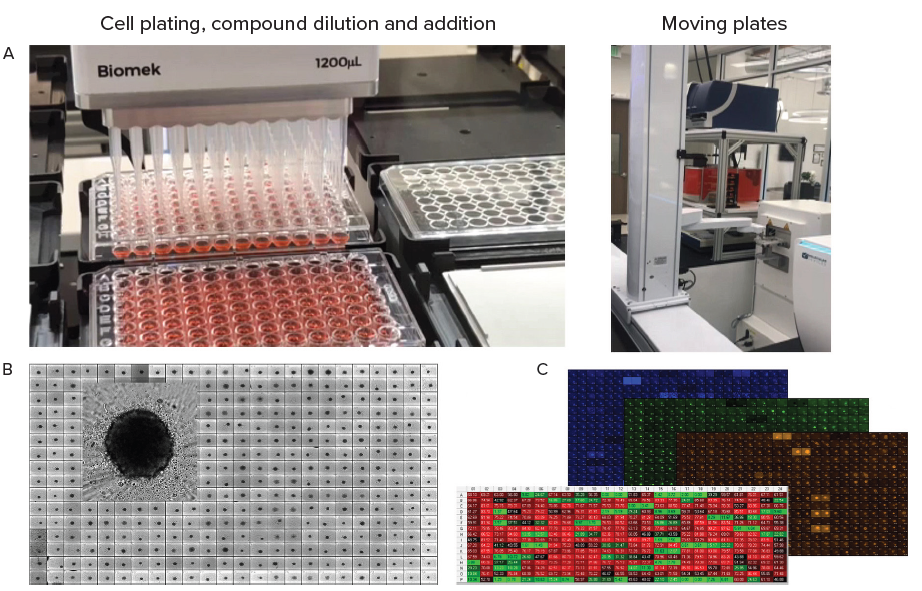
Figure 4. A. Automation of organoid culture, compound addition and staining. B. Tumoroids formed 48h after plating, TL images (10X). C. Tumoroids were treated with compounds for 5 days then stained with Hoechst dye (blue), Calcein AM (green) and EtHD (red), 10X. Organoids were imaged using confocal option, Z-stack of 15 images 10 μm apart,. Image analysis was done using Custom Module Editor (MetaXpress software) for finding organoids, nuclei, live and dead cells. Image Credit: Molecular Devices UK Ltd
Automation of cell culture, compound treatment, and imaging
For end point assays, the cells were stained with a combination of Hoechst, Calcein AM and EtHD. Cells were analyzed utilizing Custom Module Editor for complex phenotypic analysis. Tumoroid phenotypes, sizes, and density were automatically detected and characterized via AI-based image analysis.
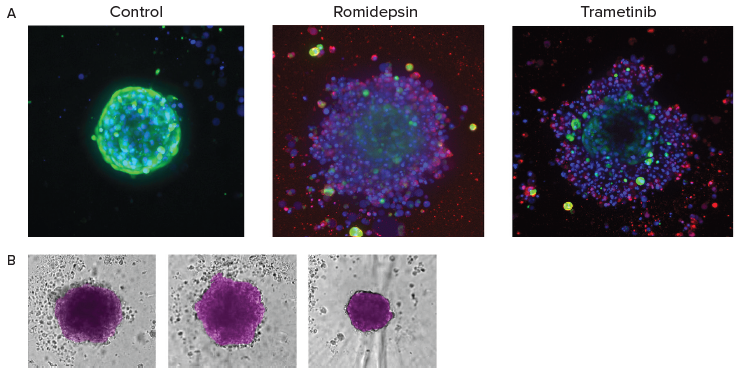
Figure 5. A. End-point analysis of fluorescent images was done using Custom Module Editor ImageXpress software. Images (maximum projection), and analysis masks shown. Multiple measurements were derived for cell scoring and organoid characterization. B. Automated monitoring and image analysis of 3D cancer microtissues was done using transmitted light images (10X) with AI-based image analysis In Carta software (analysis mask shown in purple). Image Credit: Molecular Devices UK Ltd
Conclusions
Organoid assay and compound treatment was automated via the integration of several instruments. These instruments employed in this process facilitated the automation of cell culture, maintenance, and compound treatment of 3D cellular models. The process can be used for automation and compound screening in 3D biology.
Employing confocal imaging technology in combination with 3D analysis allowed for complex, quantitative analysis of cellular content of organoids, as well as the counting and measurements of cells with different phenotypes.
These methods can be employed to evaluate phenotypic changes caused by test compounds and disease modeling.
Acknowledgments
Produced from materials originally authored by Oksana Sirenko, Zhisong Tong, Krishna Macha, and Angeline Lim from Molecular Devices LLC.
About Molecular Devices UK Ltd

Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.