Discovery of efficient anti-cancer drugs and drug combinations is essential for therapy success. Therefore, it is crucial that new methods for efficient drug efficacy testing are developed to support the discovery of new viable therapeutic targets. Being highly representative of the structure and behavior of tumors, 3D cancer cell models, such as tumoroids, are extremely valuable tools for cancer research and drug development. However, manual 3D cell culture workflows are labor-intensive, error-prone, and inconsistent, making their adoption for high-throughput drug screening cumbersome and time consuming.
To speed-up and standardize the spheroid assay, Molecular Devices UK developed 3D cell culture automation methods utilizing the CellXpress.ai™ Automated Cell Culture System to deliver automated plating, passaging, media exchange, and organoid monitoring in relation to compound treatment and endpoint assays. This study describes the automation of a colorectal cell culture workflow, where the culture and imaging of colorectal cancer 3D spheroids derived from HCT116 cell lines in U-shape low attachment plates were automated.
HCT116 cells were extended in 2D and spheroids were created after automated dispensing of the cell suspension into U-shape 96 or 384-well plates. After 48 hours, several anti-cancer compounds were used to treat the spheroids at various concentrations for 3–5 days, followed by staining and imaging.
The CellXpress.ai system was used to automatically perform cell plating, compound addition, and media exchange. During cell culture automation, the spheroids were observed using transmitted light to analyze phenotypic changes, including growth inhibition and spheroid disintegration.
For the endpoint assay, the spheroids were stained with a mixture of the Hoechst nuclear stain and viability dyes Calcein AM and EtHD. The spheroids were then subjected to imaging and analysis for spheroid size and live-dead cell scoring. Moreover, the ATP content was measured using a CellTiter-Glo assay. Luminescent read-outs were acquired using the SpectraMax® iD3 Multi-Mode Microplate Reader. A concentration-dependent decrease in ATP content was observed along with inhibition of spheroid growth, and cell death in response to anti-cancer compounds.
Automation of organoid workflow
The cell culture process can be completely automated with the new CellXpress.ai cell culture system owing to its an integrated incubator, liquid handler, and image-based decision-making. This hands-off solution takes control of demanding feeding and passaging schedules as it monitors the development of cell cultures with periodic imaging and analysis, and optimizes machine learning to launch the next processing step or troubleshoot issues.
Cell culture protocols
3D spheroids were created using HCT116 colorectal cells (ATCC) and U-shaped ultra-low attachment 96-well plates (Greiner). To culture the cells, seeding, media exchange, compound addition, and staining steps were followed, as well as imaging and analysis, using the CellXpress.ai system.
Spheroid imaging was performed during culture in transmitted light (TL) using 4x magnification. End point assay imaging was conducted using 10x magnification with florescent imaging (FL) and analysis for organoid size and intensities of viability markers predicated on staining with Calcein AM, EtHD and Hoechst nuclear stain.
Analysis in TL was completed using IN Carta® Image Analysis Software’s SINAP model and analysis in FL was run using CME analysis protocol.

Figure 1. The CellXpress.ai cell culture system components and functionality. Image Credit: Molecular Devices UK Ltd
Automation of 3D spheroid/tumoroid assay
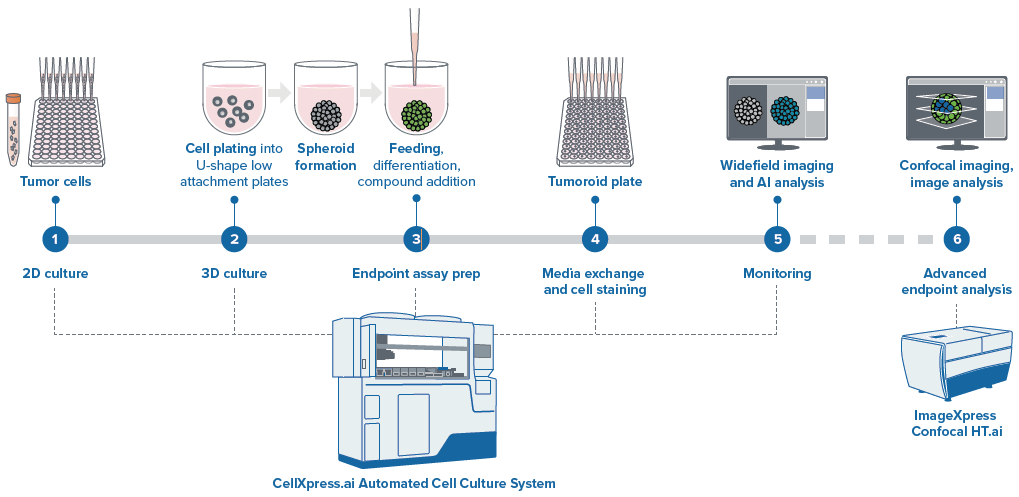
Figure 2. Schematic diagram of automated spheroid culture. Image Credit: Molecular Devices UK Ltd
Brief description of spheroid protocol
Protocol began from cells suspension with media inserted into a deep well reservoir (12 well reservoir). The cell suspension was distributed into the 96 well U-bottom low attachment plates (Greiner or Corning).
Plates were inserted into the incubator, during the incubation period, imaging was run at 12h or 24 h intervals using 4x or 10x magnification, in TL. Image analysis was performed intermittently to detect spheroids and evaluate size and density.
Compound addition: 48 hours after plating, 50 µl of media was taken out and 2x concentrations of compounds in the volume of 50 µl were introduced via the “different media” protocol.
Compounds were pre-diluted in the 96-well deep well block. Then, from a single column of the block, each compound was inserted into four columns of the 96-well plate. Each compound was introduced as a separate step that also necessitated the defining of a plate map for each compound that then triggered a compound addition step.
Staining spheroids: After three days had passed, organoids were stained with a premixed solution of three viability dyes. Staining was performed as part of the media exchange step. It should be noted that to prevent cross-contamination, tips must be discarded and cannot be re-used.
Staining incubation was run in the incubator for 1 hour. Only one washing step was required, which was done using media exchange (feeding) phase with PBS. Spheroids imaging was performed in line with the pre-defined protocol using DAPI, FITC, and TexasRed: 10–15 steps, 10–15 µm apart, with offset 50–100, Z-stacks around focus. Best focus projections were applied for the analysis.
To properly define spheroids sizes and intensities with various channels, CME analysis protocol in IN Carta® Image Analysis Software was applied. Observed shifts in spheroid area and average intensities as well as ratios of live/dead average intensities were applied to assess any compound effects. For a comprehensive phenotypic characterization of cells, it is advisable to use the ImageXpress® Micro Confocal system.
Automated spheroid culture and analysis
The spheroid workflow suits cancer spheroid assays, cardiac organoids, and neuro-spheroids. The main steps are outlined and described below:
- Cells plated into U-ULA plates
During this process, the cell suspension in media is pipetted from a deep well reservoir. 100 µL 10,000 cells are seeded into 96-well U-bottom plates (Greiner).
- Formation of spheroids
U-ULA plates are incubated for 48 h for spheroids formation and monitored using imaging techniques.
- Media exchange
Remove spent media and introduce fresh media. The protocol is calibrated for repeated media exchange every “X” hours. The full workflow protocol is configured in user-defined “phases,” including plating, feeding/monitoring, staining, and imaging. Each phase defines primary attributes (source and destination plates, pipetting protocols, volumes, media, imaging settings, and analysis protocols). Parameters can be fine-tuned to allow further optimization. Process decisions can be made automatically based on imaging analysis.
- Monitoring, imaging
User-defined periodic imaging of the plate combined with real-time image analysis supported by a pre-trained deep-learning-based model or customized protocol. Data analytics tools allow users to view the growth of organoids over time. Staining organoids and washes was completed by following the “media exchange” protocol step. FL imaging of spheroids was conducted after treatment with compounds and staining with Calcein AM, MitoTracker, and Hoechst nuclear stain.
- Staining and compound treatment
Spheroids were imaged using CellXpress with the DAPI, FITC, and Texas Red channels, with a 10x or 4x objective. Image analysis was carried out using CME (IN Carta).
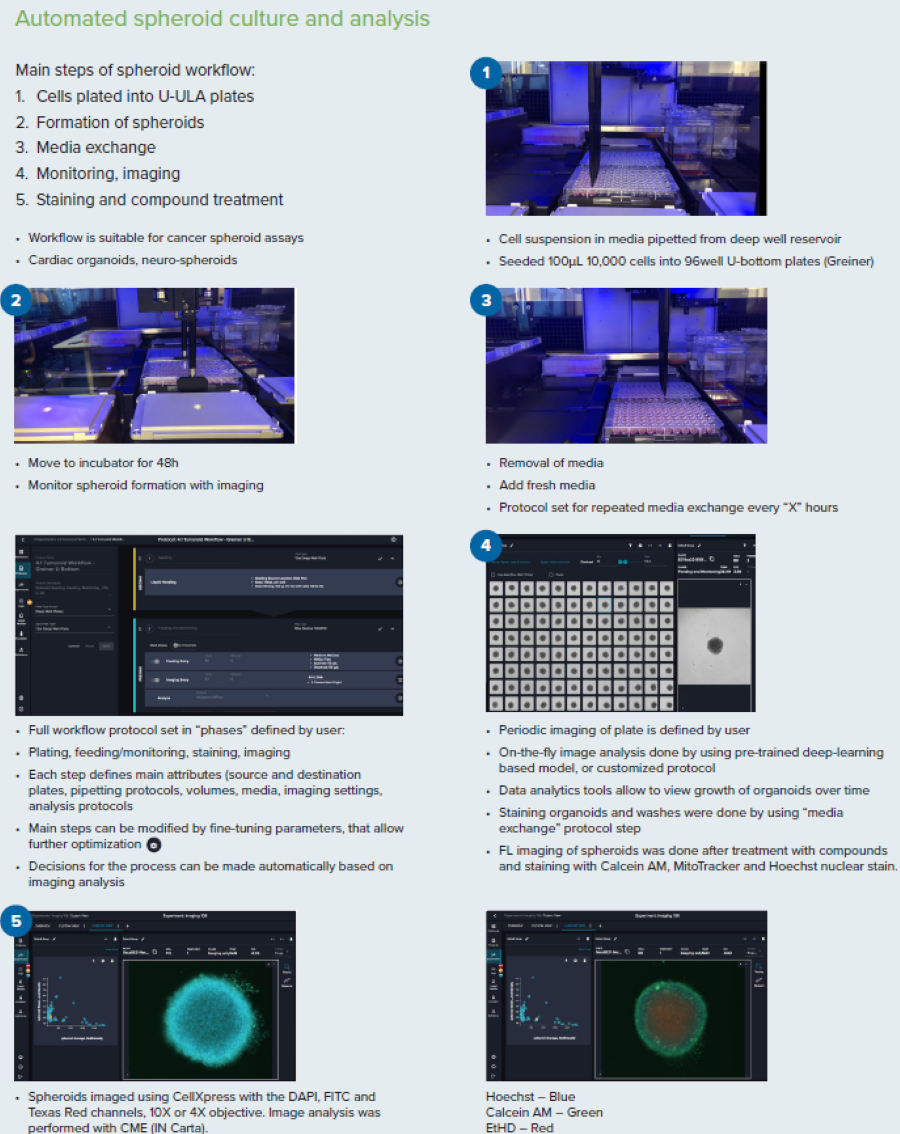
Figure 3. Steps for the spheroid culture and analysis workflow. Image Credit: Molecular Devices UK Ltd
Results
Phenotypic evaluation of compound effects
It has been demonstrated here that the workflow enables the formation spheroids/tumoroids using HCT116 colorectal carcinoma cells as an example. The CellXpress.ai instrument helped form and maintain spheroids with an automated culture process.
After treating the spheroids for three days with anti-cancer compounds (staurosporine, doxorubicin, paclitaxel), they were stained with cell viability dyes Calcein AM (live cells) and EtHD (dead cells), and nuclear stain Hoechst.
Spheroids were imaged using viable fluorescent channels DAPI, FITC and Texas Red, using best-focus projection images from Z-track of images captured at 10x magnification.
Fluorescence intensities and the ratios of live/dead staining were then evaluated. As displayed in Figure 5, there is a decrease of the ratios of live/dead marker intensities with increasing concentrations of compounds.
The decrease in cell viability was assessed by CellTiter Glow reagent (measure of ATP content). It was observed that a decrease in ATP content with increase of compound concentrations occurred.
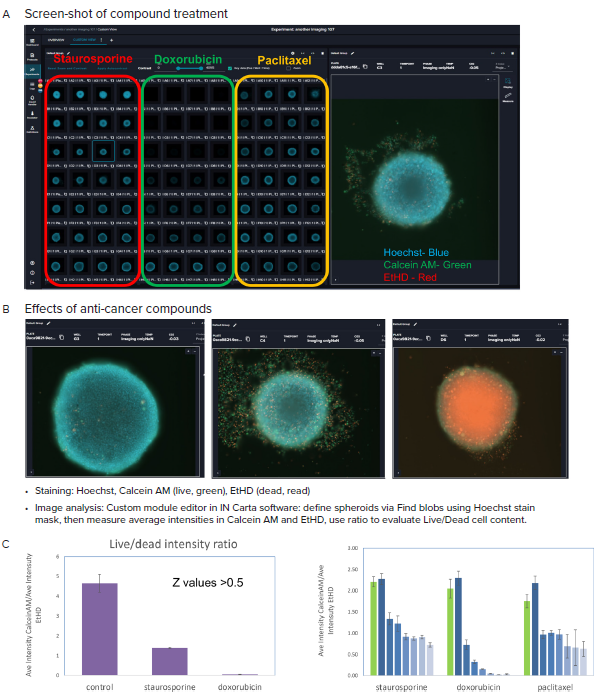
Figure 4. A, B Representative images (10X) of organoid culture taken after treatment with indicated compounds and staining. C. Graphical presentation of spheroid analysis: Ratios of the Ave Intensities of spheroids for Calcein AM and EtHD, maximum concentrations, and across concentration range. Averages and STDEV calculated from quadruplicates. Image Credit: Molecular Devices UK Ltd
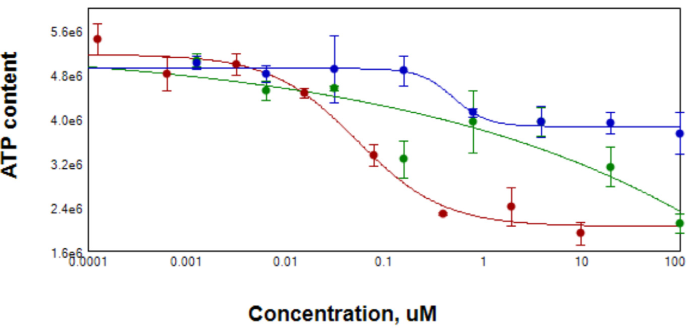
Figure 5. After compound treatment for 3 days, spheroid samples were tested for ATP content using CellTiterGlo reagent for 3D samples. EC50 for Staurosporin (red) was 0.0 μM, for Paclitaxel (blue) 0.5 μM. Data for Doxorubicin (green) were ambiguous due to the possible contribution of compound into Lumi signal. Image Credit: Molecular Devices UK Ltd
Summary
There is a significant need to develop more effective and personalized approaches to cancer treatment. The transition from 2D to 3D cell biology has game-changing potential in cancer modeling and drug screening applications. This is due to the fact 3D models closer resemble tissue structure and functionality with more predictive responses to drug effects.
However, 3D cell culture protocols are vastly complex and somewhat restricts the large-scale culturing of cell lines and their widespread biomedical applications. However, CellXpress.ai Automated Cell Culture System simplifies the entire process, from cell culture to assay set-up, screening, cell culture imaging, and data analysis.
It can be concluded that automation facilitates reproducible 3D cell culture workflows at scale, significantly boosting confidence in the drug screening readouts and creating deeper insights for high throughput drug discovery and precision medicine applications.
About Molecular Devices UK Ltd
Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.