The effectiveness of non-clinical drug safety predictions is enhanced by the adoption of three-dimensional (3D) cellular models. 3D bioprinting enables the generation of complex models with spatial control and a variety of matrices, allowing the formation of complex tissue structures.
This article will delve into a method for the automated generation of 3D cellular liver models using a multi-tool robotic device with liquid handling. This method allowed for the automated bioprinting and high-content imaging of liver cells within a collagen matrix and was used for compound testing and toxicity effect evaluation in liver cells.
For example, in an assay using bio-printed structures, HepG2 cells were resuspended in collagen I with micro-structure printed channels, enabling better media diffusion. Using a printing tool and pipettor, the robot printed pluronic acid into wells of a multi-well plate to create the 3D structures needed for channel formation, then dispensed the cell mix with collagen to create a thick 3D cell layer.
Benefits:
- Consistent 3D bioprinting and dispensing with flexible built-in assays and multiple customizable tools.
- Long-period monitoring and high-content imaging for 3D cellular models.
- cGMP and user-friendly interface for accessible building of complex organ-specific models.
Materials
BAB400
The BioAssemblyBot® 400 (BAB400) by Advanced Solutions is a cGMP-certified multi-tool and multipurpose platform for the construction of 3D models. Using the tissue structure information modeling (TSIM) software, this tool was employed to design 3D structures for 3D printing using the ambient tool and Bioapps Maker. This system was also used to automate dispensing, imaging, and maintenance of the 3D models.
ImageXpress Micro Confocal system (Imaging)
Transmitted light (TL) and fluorescent images were attained using the ImageXpress® Micro Confocal HighContent Imaging System from Molecular Devices, using MetaXpress® High-Content Image Acquisition and Analysis Software.
Z-stack images were acquired with 4X or 10X objectives for the liver tissue models using confocal mode. MetaXpress software was used for all analyses.
Printing and cell model preparation
HepG2 human liver cancer cell line cells were cultured following the ATCC protocol. The equipment facilitated media addition, exchanges, and collection. Utilizing the ambient 3D print tool, hexagonally arranged Pluronic pillars were then created.
The cells were lifted off the plate surface, spun, and mixed with collagen media (2 mg/mL) to obtain 20 million cells/mL after the pluronic pillar arrays were printed and warmed to 37 °C for 30 minutes in an incubator.
Then, 50 μL of the collagen/cell suspension at 8 °C was introduced to the wells with pillars. The pillars were then removed using temperature-based dissolution and washed twice with the pipette tool, leaving a structured, cell-dense, 3D liver cell construct remaining.
The 3D HepG2 models were cultured and monitored daily via transmitted light imaging. Later, these models were treated with a panel of drugs of known liver toxicity, including chloroquine, haloperidol, pimozide, taxol, doxorubicin, cisplatin and mitomycin.
This treatment took place on the fourth day of model formation, and imaging was performed 48 hours later. Cells were stained with viability dyes and imaged using a confocal imaging system for the endpoint measurements.
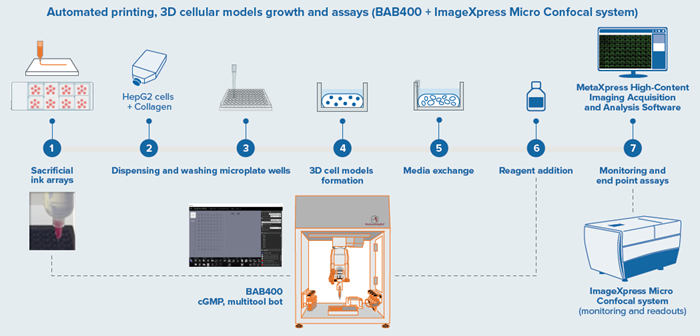
Figure 1. BAB400 3D printing and dispensing workflow integrated with Imager for endpoint assays. Image Credit: Molecular Devices UK Ltd
Results
Culture of HepG2 cell models
Using TSIM design software, the BAB400 was successfully employed to develop HepG2 cell models in 96 well formats, as shown in Figure 2.
TSIM software was used to design the array patterns in 96-well plate format. These were then printed and laid with cells mixed in collagen I. These were monitored for four days before further drug treatments and imaging.
Drug treatment and imaging
Multiple 96-well plates were observed until day four and then treated with multiple drugs for 48 hours. The cells were then stained for live and dead counts and analyzed to obtain viability data.
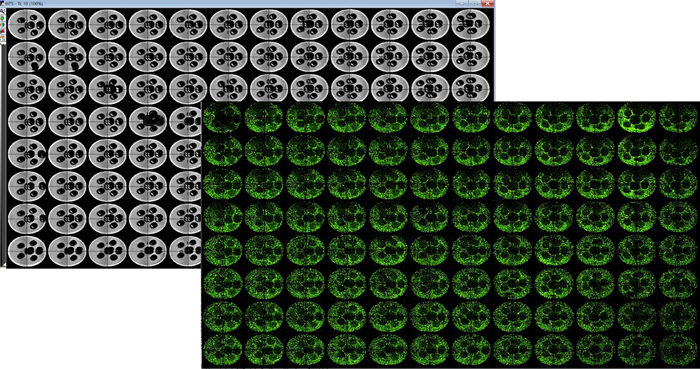
Figure 2. TL image of 3D printed pluronic pillar arrays captured using ImageXpress Micro Confocal system (4X) (left–grey panel). Plate representations of uniformly seeded HepG2 cells in collagen I after pluronic pillars were washed (right – green panel). The holes in the dense collagen matrix enabled for better exchange of nutrients and gases. This eventually prevents the cells in the center of the matrix from undergoing necrosis. Image Credit: Molecular Devices UK Ltd
Following drug treatment on the fourth day of observation, the cells were stained for live and dead counts and analyzed to obtain viability data.
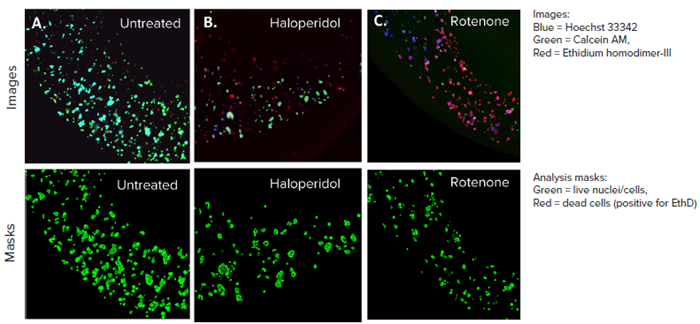
Figure 3. HepG2 3D cell model confocal images, 4X single site image (top panel), and their representative live analysis masks generated by MetaXpress software (bottom panel) A) Untreated control, B) Haloperidol (20 μM), C) Rotenone (4 μM). The control wells had more HepG2 cells and lower cell death, whereas the treated cells had more red-stained cells and a lower number of live cells, indicating cell death. Image Credit: Molecular Devices UK Ltd
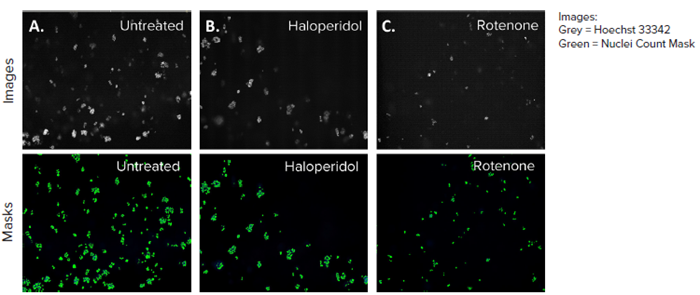
Figure 4. HepG2 3D cell model DAPI images, 10X single site image (top panel), and their representative nuclei count analysis masks generated by MetaXpress software (bottom panel) A) Untreated control, B) Haloperidol (4 μM), C) Rotenone (4 μM). The DAPI mask counted the number of nuclei present in each site and the control (A) had the most nuclei owing to no toxicity. Image Credit: Molecular Devices UK Ltd
Drug response and imaging data
The same drug treatments were also performed on 2D HepG2 cultures and the difference in dose response with 3D HepG2 cell models was compared. Compound effects were observed at lower concentrations in the 2D systems compared with 3D.
Imaging of models was performed using confocal, 4x and 10x, and projection images were analyzed using live-dead analysis. Image analysis using Custom Module Editor in the MetaXpress software can be employed to locate cells, subcellular structures, and treatment responses.
This entire workflow may be automated using the BAB400 BioApps Player solution. Figure 5 displays a composite image of three stains: Calcein AM, Ethidium homodimer – III (EthD) and Hoechst 33342.
These images are a representation of all the wells, with control wells appearing in brighter green stains and fewer red stains, indicating more live cells and fewer dead cells. The compound effects resulted in increased uptake of EthD, representing cell death phenotype.
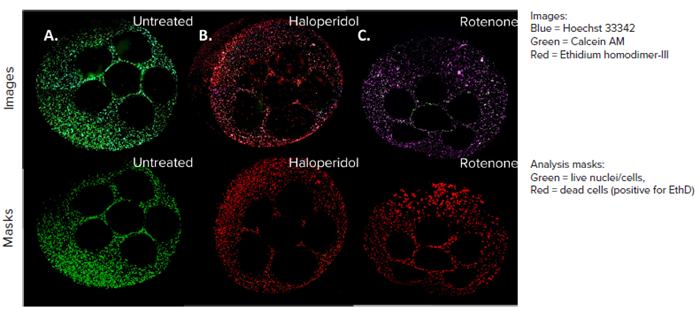
Figure 5. HepG2 cell model confocal images, 4X all sites image (top panel), and their representative live dead analysis masks generated by MetaXpress software (bottom panel). A) Untreated control, B) Haloperidol (100 µM), C) Rotenone (100 µM). Image Credit: Molecular Devices UK Ltd
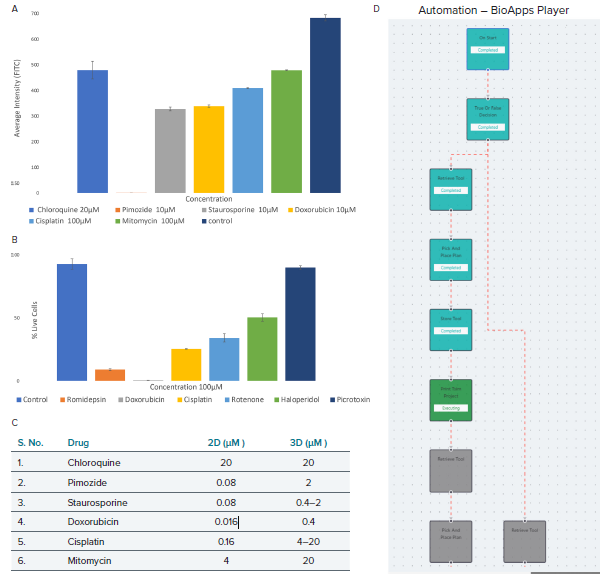
Figure 6. The HepG2 cell model projection images were analyzed using live-dead analysis. A) The average intensity of fluorescence against the various drug dose effects (seeding density 7 million cells/mL). B) Live cell percentage of treated cell models against the highest dose of drugs, i.e., 100 μM and cell seeding density of 20 million cells/mL. C) Comparison between the 2D and 3D HepG2 cell models indicating the minimum dosage in μM required for phenotypic response. D) BioApps Player workflow for a basic print workflow which allows for full automation. Image Credit: Molecular Devices UK Ltd
Summary
Comparison of 2D and 3D HepG2 assays revealed compound effects at lower concentrations in 2D systems compared with 3D, demonstrating differences in compound responses between 2D and 3D assay formats.
The limitations of using HepG2 cells for toxicity assessment are linked to the fact that cells continued to proliferate in the culture. As a result, most of the effects were demonstrated with anti-proliferative compounds. Employing non-proliferating, primary or iPSC-derived liver cells would give a more predictive model for the evaluation of toxicity effects in the liver.
Conclusions
The results discussed demonstrate the value of forming liver 3D bio-printed models using an automated method. The benefits of employing imaging and data analysis methods and descriptors to gain more information about complex compound effects in 3D-printed and cell-tissue-engineered cell models are also evidenced.
Improvements in accuracy and throughput can be achieved by implementing automation. The process discussed here can be fully automated by integrating several instruments to provide automated cell culture, maintenance and differentiation of 3D cellular models. These can then be used for compound screening in a variety of assays.
Acknowledgments
Produced from materials originally authored by Prathyushakrishna Macha and Oksana Sirenko from Molecular Devices LLC and Sarah M. Moss and James B. Hoying from Advanced Solutions Life Sciences.
About Molecular Devices UK Ltd 
Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.