Patient-derived organoids (PDOs), including those from tumors, are implemented as predictive models of patient responses to drugs and therapies. Incorporation of the BioAssembly™ platform has been demonstrated to optimize the complex and indispensable three-dimensional (3D) tissue models for drug screening.
This agile, state-of-the-art automation solution integrated the BioAssemblyBot® (BAB), BioStorageBot™ modular incubator and BioApps™ intuitive workflow control software with confocal imaging into the workflow.
Following the automated preparation, feeding, drug treatment and image acquisitions, image sets were analyzed to generate high-content assessments of PDO responses to the drugs.
The BAB platform improves the accuracy, efficiency and reproducibility of dispensing PDOs into multiwell plates by employing highly tunable operation parameters, such as pipetting speed, minimizing organoid fragmentation and non-uniform plating.
In the study discussed in this article, an automated PDO drug screen involving automated imaging and High Content Analysis (HCA) with the BAB platform was performed.
Figure 1 shows the workflow, which involved assessing the response of colorectal cancer PDOs from two different patients to the drugs trametinib and adavosertib at four different doses.
Up to 76 image features were extracted from the image sets generated during the automated workflows and were evaluated for drug- and dose-specific responses. The automated PDO assay with HCA was demonstrated to be capable of identifying the differences between PDOs treated with different drugs.
Benefits of PDO and workflow automation
- Automation enhances the quality, throughput, and consistency of assays.
- Applicable to a variety of PDO types and systems.
- Easy-to-use BioApps software integrates and controls all components.
- BioAssembly’s flexible and easy-to-use platform is conducive to automated imaging and high-content analysis.
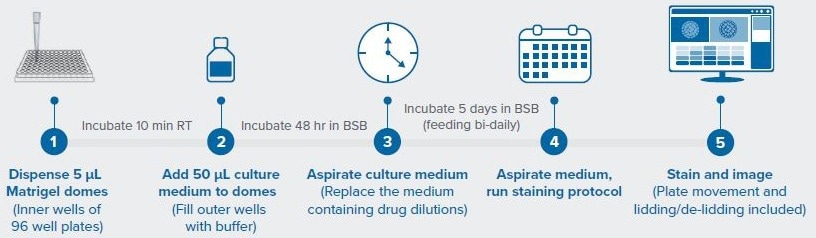
Figure 1. Workflow for automated PDO drug screen. Image Credit: Molecular Devices UK Ltd
Materials and methods
Colorectal cancer (CRC) PDOs from two different donors were provided by Molecular Devices. These were suspended in 80 % Matrigel® and dispensed into a 96-well plate using the BAB and pipette hand. A dome was formed by dispensing 5 µl into the center of each well. Following gelling, the BAB introduced a culture medium to each well and transferred the plate to the modular BioStorageBot incubator.
On the second day of culture, the BAB changed culture media and introduced a serial dilution series of trametinib (MEK1/2 inhibitor) or adavosertib (tyrosine kinase WEE1 inhibitor) to the wells.
Cultures were imaged using an integrated confocal imager after seven days, following a Hoechst staining protocol. Offline, image analysis using IN Carta software was performed on the maximum projection images. Multiparametric analyses were executed using StratoMineR from Core Life Analytics.
Following imaging, a CellTiter-Glo® 3D assay was performed to measure Adenosine triphosphate (ATP) production. All steps of the PDO assay, including the automated imaging protocol, were controlled by a user-defined PDO BioApp software module.
Results and discussion
Properly dispensing PDOs in Matrigel can be challenging as to form a dome, it must be deposited exactly in the center of the well. The dome is necessary to prevent Matrigel from forming a meniscus at the well edge, which can complicate imaging.
Considerable practice is required for a scientist to achieve a high success rate of dome formation. Incorporation of the BAB is shown to improve the dome formation rate by 10 %, as well as reduce dome dispensing time by 50 %, compared with no automation, as shown in Figure 2.
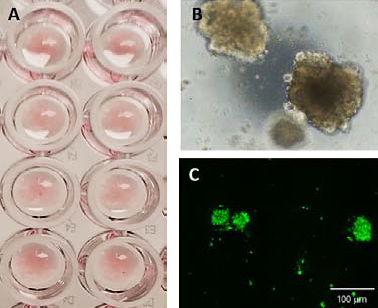
Figure 2. CRC PDOs seeded in Matrigel domes in a 96 well plate (A). PDOs after 7 days of culture (B) phase contrast image, and (C) calcein AM. Image Credit: Molecular Devices UK Ltd
After two days, serial dilutions of trametinib and adavosertib were introduced to wells. The PDOs were incubated for a further five days, after which an end-point viability assay was carried out.
CellTiter-Glo 3D, a tool for measuring ATP production as an indirect measure of cell viability, demonstrated clear differences between the highest and lowest drug concentrations, as well as the untreated controls, as shown in Figure 3. In spite of this, no discernable change in organoid diameter in response to the inhibitors was observed, as shown in Figure 4.
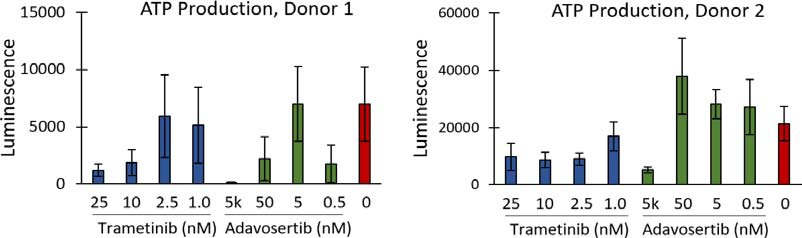
Figure 3. ATP production of PDOs, as measured by CellTiter-Glo 3D, performed at the end of the assay. Image Credit: Molecular Devices UK Ltd
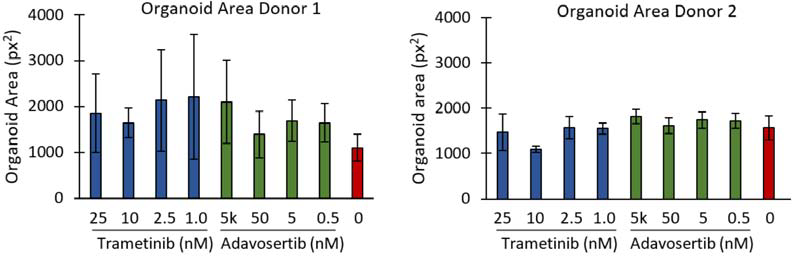
Figure 4. Area of PDOs treated with different concentrations of trametinib or adavosertib, compared to untreated controls. Plates were seeded with PDOs from one of two different donors. Bars are mean ± SD. Image Credit: Molecular Devices UK Ltd
Deep-learning-based segmentation in IN Carta was utilized to extract features such as area, intensity, form factor and texture from the data set, which were then analyzed using StratoMineR’s cloud-based advanced analytics.
In this study, focus was given to principal component analysis (PCA) and Euclidian distance analyses as baseline readouts for the automated workflow. PCA of up to 76 features found differential responses by the PDOs to the inhibitors, as shown in Figure 5.
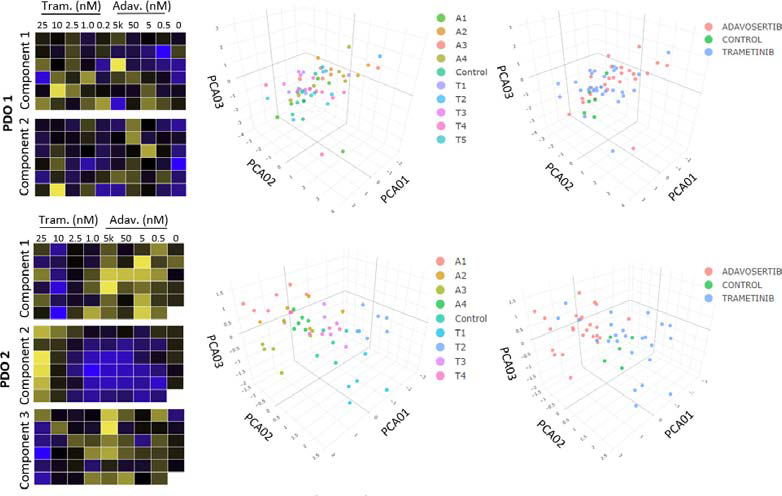
Figure 5. Heat maps and principal component analysis (PCA). For Donor 1 (PDO 1), 2 components are shown, which show limited clustering within or between drug concentrations. Donor 2, however, had clear clustering in components 1, 2, and 3, within each drug compared to controls. Image Credit: Molecular Devices UK Ltd
PDOs from Donor 1 were shown to have limited response to the two inhibitors, while PDOs from Donor 2 displayed clear differences in inhibitor responses in a dose-dependent manner.
Evaluation of the hit rate via phenotypic distance mapping confirmed the relative unresponsiveness of Donor 1 PDOs to the inhibitors, with a hit rate of 31.5 %, demonstrated in Figure 6. On the contrary, both inhibitors showed a significant impact on Donor 2 PDOs, with a hit rate of 100 %.
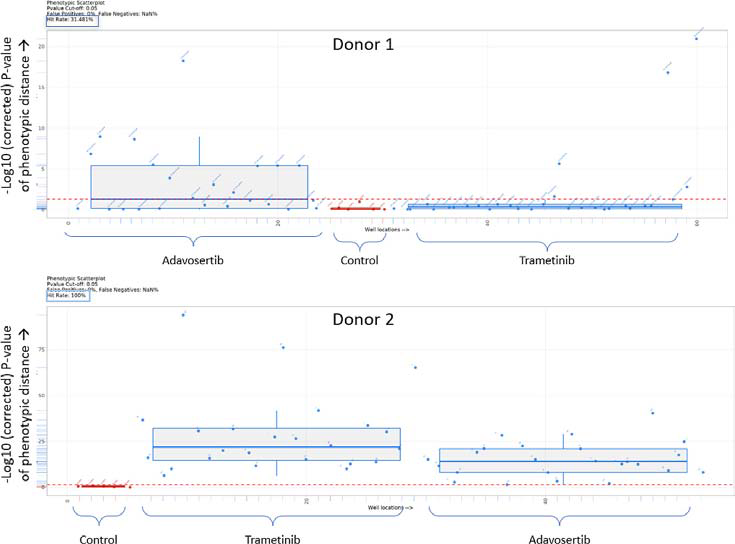
Figure 6. Box plots showing the Euclidian distance from the median of the control (untreated) for PDO 1 and PDO 2. Everything above the red dotted line has a p value <0.05. Image Credit: Molecular Devices UK Ltd
Conclusions
This study demonstrates the effectiveness of a fully integrated automation platform for screening the effects of therapeutic compounds on patient-derived tumor organoids. The automated PDO assay was able to identify patient-specific differences in PDO responses to selected signaling inhibitors via high-content image analyses.
While a more detailed study to establish measurements of consistency, accuracy and precision is required, the automated workflow studied here was capable of producing results comparable to those obtained by a skilled scientist in approximately half the time and with improved efficiencies.
The combination of automated PDO formatting, culturing, treating and imaging with high-content analysis represents a powerful, simple and highly adaptable method for high-throughput drug screening.
Acknowledgments
Produced from materials originally authored by Hannah A. Strobel from Advanced Solutions Life Sciences, Victoria Marsh and Angeline Lim from Molecular Devices, and Maria R. Oyaga from Core Life Analytics.
About Molecular Devices UK Ltd 
Molecular Devices is one of the world’s leading providers of high-performance life science technology. We make advanced scientific discovery possible for academia, pharma, and biotech customers with platforms for high-throughput screening, genomic and cellular analysis, colony selection and microplate detection. From cancer to COVID-19, we've contributed to scientific breakthroughs described in over 230,000 peer-reviewed publications.
Over 160,000 of our innovative solutions are incorporated into laboratories worldwide, enabling scientists to improve productivity and effectiveness – ultimately accelerating research and the development of new therapeutics. Molecular Devices is headquartered in Silicon Valley, Calif., with best-in-class teams around the globe. Over 1,000 associates are guided by our diverse leadership team and female president that prioritize a culture of collaboration, engagement, diversity, and inclusion.
To learn more about how Molecular Devices helps fast-track scientific discovery, visit www.moleculardevices.com.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.