With each heartbeat, blood courses through your body. Some traverse arteries from the lungs back to the heart, and some navigate through capillaries in the thinnest layers of your eyelid, almost single-file, one frisbee-shaped cell at a time.
As blood circulates, it exerts force on everything it touches. This force is felt in every organ of the body. However, when scientists aim to recreate an organ outside of the body using technologies such as Organ-on-a-Chip (OOC), the absence of these blood flow forces can lead to lab-grown organs and tissues with diminished physiological relevance.
This can result in organs that do not function the same way as their counterparts in the human body or deliver experimental data representative of them.
For the future success of OOC technology, also known as known as a Microphysiological System (MPS), the incorporation of fluid flow to mimic the circulatory system is crucial.
What is flow?
In simple terms, flow refers to the movement of fluid through a vessel. Whether it is blood moving through a broad artery or a tiny capillary, as a fluid moves, it exerts forces against the vessel walls, known as shear forces.
Research indicates that the shear stress from blood flow is crucial for the proper development and functioning of tissues and organs.
To comprehend the diverse shear stresses that blood flow imposes on tissues, bioengineers and scientists must consider the viscosity of blood, the sizes of different blood vessels it flows through, and the flow profile - whether it is smooth or pulsatile.
Taking these factors into account helps them understand fundamental processes in physiology, such as the rate at which nutrients can be transported from the blood to the tissue cells and how efficiently waste products are removed. All these calculations are essential when designing OOC models.
How do Organ-on-a-chip platforms regulate flow?
Inspired by the human heart, OOC bioengineers utilize pumps to propel fluid through their systems, mimicking the natural forces of blood coursing through an organ. However, not every organ in the body undergoes the same force of blood flow.
For instance, major blood vessels like arteries experience high and rapidly changing forces, while narrow capillaries within tissues encounter a much more consistent and gentle set of flows and forces.
Consequently, depending on the organ or tissue being replicated, the OOC system should provide either a robust and pulsatile flow or a slower, steadier flow.
Many pumps employed in OOC systems generate pulsatile flow, which is effective for modeling arteries. However, if the aim is to model other organs, such as the liver, which experiences a much steadier flow, the trick is to damp out these pulses.
How does incorporating flow into Organ-on-a-chip technology improve upon in vitro 2D culture?
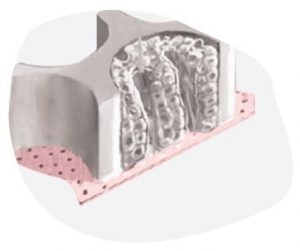
Image Credit: CN Bio Innovations Limited
A 3D OOC culture already represents a significant step closer towards recapitulating an organ’s microarchitecture compared to standard 2D in vitro culture. By incorporating flow, 3D models edge ever closer to mimicking the functionality of living human organs.
In CN Bio’s human Liver-on-a-Chip (also known as Liver MPS), the role of flow is crucial for the formation and maintenance of liver tissues.
Studies have demonstrated that liver tissue exposed to continuous flow, often called perfusion, exhibits increased viability and higher production of liver-specific proteins such as albumin.
These tissues can also be cultured for extended periods underflow (up to four weeks), which unlocks possibilities for studying chronic drug exposure, disease modeling, and the effectiveness of potential therapeutics over static cultures.
The 3D liver tissues, generated by CN Bio’s PhysioMimix®, recreate the liver’s microarchitecture, maintain their phenotype for up to 4 weeks, and deliver clinically translatable biomarkers such as AST.
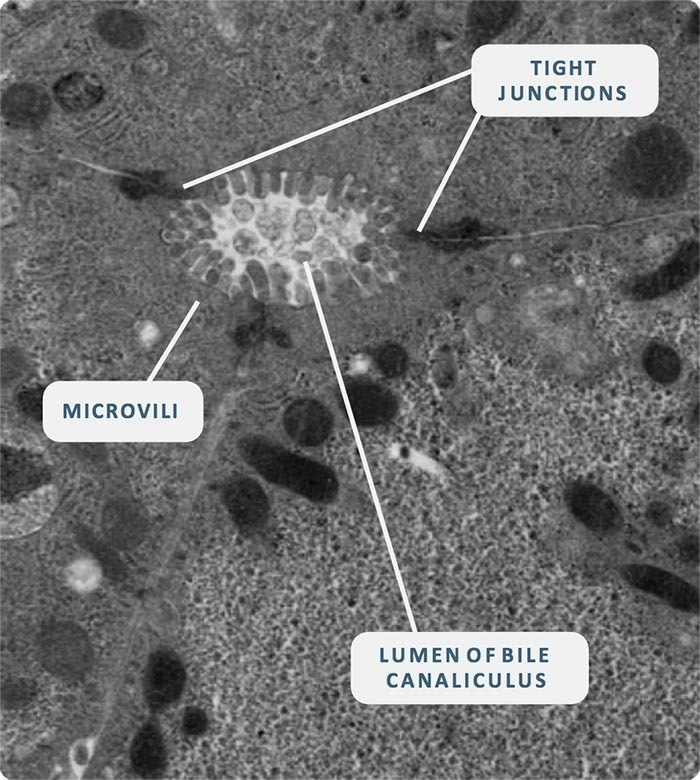
Image Credit: CN Bio Innovations Limited
Flowing from organ to organ
In the human body, blood does not merely flow to and from one organ at a time. It circulates through various organs, transporting nutrients, drugs, signaling proteins, and metabolites from one part of the body to the next.
An illustration of this is the connection between the gut and liver. Recently, CN Bio has developed a human multi-organ Gut/Liver model that replicates blood flow between these organs. With these systems facilitating communication between organs and tissues, akin to the interactions in the body, the potential is substantial. This advancement enables scientists to gain a deeper understanding of how drugs are absorbed, interact with, and influence multiple organs at the same time.
For instance, connecting liver tissue with another organ presents an opportunity to explore reactive metabolite-driven toxicity. Introducing circulating immune cells into the mix allows researchers to investigate how inflammation can mediate toxicity. The Gut/Liver model can also simultaneously assess drug absorption and metabolism and, therefore, is ideal for assessing the effects of first-pass metabolism and establishing drug bioavailability, which are crucial considerations for orally administered medicines.
As drug dose is intrinsically linked to efficacy and safety, early insights are essential. This effect, previously measured using animal models and clinical trials, can now be explored in the laboratory. By studying how much drug is converted to inactive forms before reaching the systemic circulation (and thereafter the desired target organ), in vitro estimations of drug bioavailability enable more informed decisions about which drugs to take forward and the optimal dose range to use before in vivo animal and first-in-human studies to reduce the risk of halted trials.
While possibilities seem vast, the challenges are equally extensive. For example, consider the emulation of blood, specifically the task of media selection.
While using tissue-specific media is crucial for culturing healthy and phenotypically functioning organs in vitro, linking organs together poses a dilemma. One media type may facilitate the growth of one organ but be harmful to another.
To solve this, it is important to delve into physiological data, sorting through effects and carefully adding or eliminating different media constituents. The next challenge involves skilful bioengineering to establish physiologically relevant flow rates and cardiac output within each organ and between different organs in the model.
CN Bio confronts these challenges head-on, using its unique expertise and experience to develop multi-organ models that enhance the accuracy of drug discovery and development.
Traditionally, drug discovery scientists had to rely on simplistic in vitro and animal models with their well-known limitations alongside computer simulations. Now, organs and tissues made of human cells can communicate in the laboratory for many weeks, providing answers to important questions.
CN Bio’s bioengineers have enabled OOC technology to more accurately replicate how organs function in the human body, allowing scientists to gain a more comprehensive understanding of a drug's effects before in vivo animal studies such that only the most promising drugs pass through, and the numbers of animals required is reduced. Their models also provide a viable path forward for the development of molecules targeting human-specific targets, pathways and processes where animal use is less suited.
Through the delivery of lab results that translate into human outcomes, CN Bio’s advancements are rapidly closing the gap between 2D in vitro cell culture and clinical research to drive the faster development of safer and more efficacious therapeutics.
About CN Bio
CN Bio is a leading organ-on-a-chip (OOC) company that offers a portfolio of products and contract research services to optimize the accuracy and efficiency of bringing new medicines to market. With more than a decade of research and development experience, they aim to transform the way human-relevant pre-clinical data is generated through the development of advanced in vitro human organ models.
CN-Bio's PhysioMimix® Core microphysiological system (MPS) enables researchers to recreate human biology in the lab and is the only microphysiological system with validated performance across single-, multi-organ, and higher throughput configurations. This easy to adopt, adapt and scale technology bridges the gap between traditional cell culture and human studies, to support the development of safer and more efficacious therapeutics, whilst reducing the dependence on animal model usage.
CN Bio’s portfolio of products (MPS, 3D validated cells, consumable plates) and services support researchers that require reliable, data-rich, in vitro studies, to uncover novel mechanistic insights into drug or disease mechanism of action.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.