There is an increased prevalence of diabetes, metabolic syndrome, and obesity. As a result, Metabolic dysfunction-associated liver disease (MASLD) is now the most common chronic liver disease in developed countries.1
MASLD (previously known as Non-alcoholic fatty liver disease (NAFLD)) encompasses several pathologies, spanning from benign hepatic steatosis to Metabolic dysfunction-associated steatohepatitis (MASH), which can result in cirrhosis and liver cancer. As there is only one regulatory-accepted drug for MASH, improved preclinical models are needed to understand this multifaceted disease.
There is also an increased awareness of the potential risk factors for drug-induced liver injury (DILI) due to the underlying metabolic condition. There are two ways that DILI in MASLD patients can be exacerbated. Some drugs aggravate pre-existing MASLD and cause more elaborate intracellular lipid accumulation. More frequently, drugs induce liver injury and acute hepatitis.2
A higher risk of drug-induced acute hepatitis in obesity is associated with the increased activity of numerous cytochromes P450 (CYPs), which augment the generation of toxic metabolites.2 When excessively generated, reactive metabolites can cause hepatic oxidative stress, cytolysis, and severe mitochondrial dysfunction.
The mechanisms underlying these processes are not well understood. Adverse drug responses caused by fatty liver are increasing in regularity due to the continual growth of the obesity epidemic. CN Bio has developed an advanced in vitro model that explores the mechanisms and relationships that link DILI and MASLD.
Aim
Utilizing a microphysiological system (MPS), CN Bio has developed a complete human perfused in vitro MASLD model, employing primary human hepatocytes (PHH) cultured in 3D to mimic the liver microarchitecture. For up to four weeks, cells were cultured with high concentrations of free fatty acids to induce intracellular triglyceride (fat) accumulation and mimic hepatic steatosis.
In this model, cells were assessed for fluctuations in CYP activity, and the effects of known hepatotoxicants were evaluated when dosed at or around IC:50 concentrations.
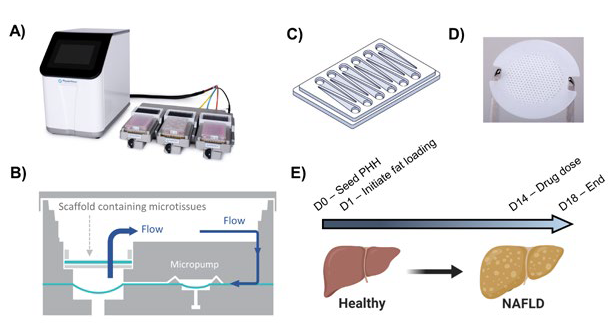
Figure 1. Human in vitro MPS MASLD model. A) The in vitro model utilizes the PhysioMimix™ cell culture system, which uses open well plates for culturing primary liver cells in 3D on an engineered scaffold. B) Schematic representation of individual culture well of a Liver-12 plate. C) Schematic of a Liver-12 multi-chip plate. D) The scaffolds held within each well on the plate are continually perfused with cell culture medium throughout the experiment to provide biomechanical stimuli and oxygen provision (3). E) The MASLD model is generated by culturing PHH in high-fat medium for up to 28 days. For studying DILI, compounds were dosed after 14 days of fat loading. Image Credit: CN Bio
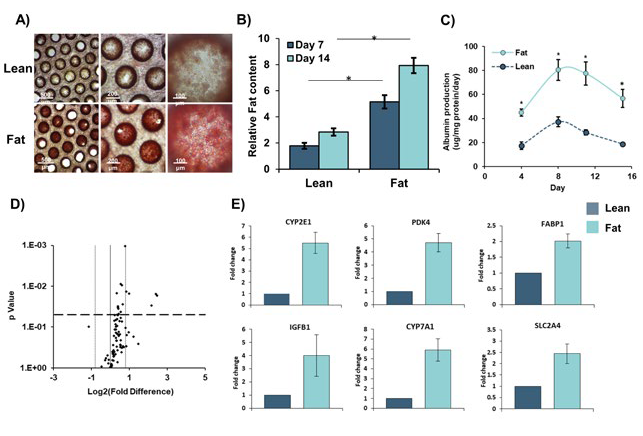
Figure 2. Primary Human Hepatocytes accumulate intracellular fat over time. Those cultured in fat media show altered gene expression profiles. PHH were cultured for 14 days under fat and lean conditions. Fat loading was measured by Oil Red O staining of microtissues and gene expression was compared using Liver RT2 Profiler PCR Arrays. A) Cells were imaged for intracellular fat accumulation and B) quantified by absorbance at 510 nm and normalized to total protein content. C) Albumin production was quantified by ELISA and gene expression changes between lean and fat cultures D) were defined by a fold change >1.8 and P = <0.05. E) Fold change in expression in fat vs lean condition of key genes. Data are mean ± SEM from 9 independent cultures (three donors per condition and n=3 per donor); * = P < 0.05. Data taken from previous publication Kostrzewski et al. 2017 (3). Image Credit: CN Bio
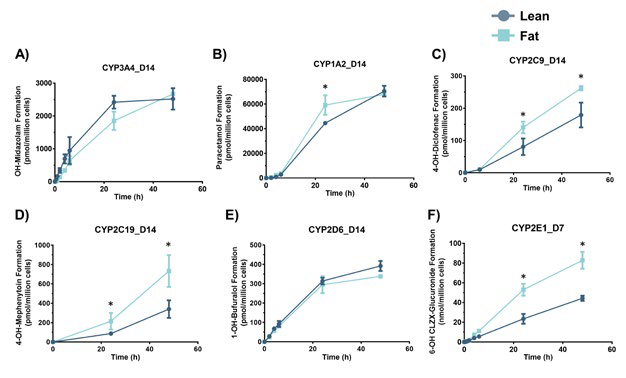
Figure 3. Hepatocytes in the MASLD model have altered metabolic activity. PHH were cultured for 14 days under fat conditions and then dosed with probe compounds to assess the metabolic activity of key CYP-450 enzymes. The activity of each enzyme was determined by the production of phase I metabolites over 48 hours. The presence of the metabolites was quantified using LC-MS and a standard curve of the metabolite. All compounds were dosed at day 14 except for CLZX (CYP2E1 target) which was dosed at day 7. All data are a minimum of n = 3. * = P < 0.05. Image Credit: CN Bio
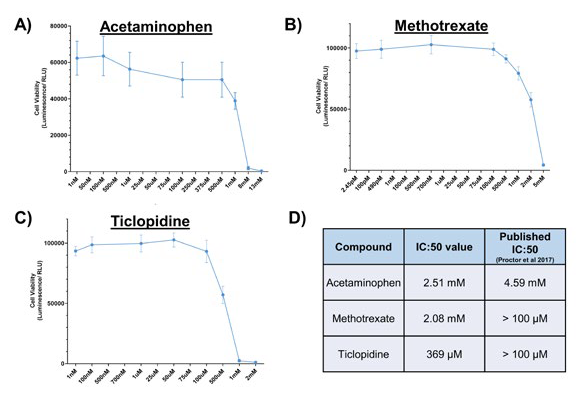
Figure 4. Determining IC:50 concentrations for compounds known to cause DILI. PHH were cultured for 72 hours on collagen-coated 96-well plates, under lean media conditions. They were exposed to varying concentrations of A) Acetaminophen, B) Methotrexate, and C) Ticlopidine for 48 hours before cell viability was assessed using Cell Titre Glo. D) IC:50 values for each compound were generated and compared to published data (4). Data shown are a mean ± SD and a minimum of N=4. Image Credit: CN Bio
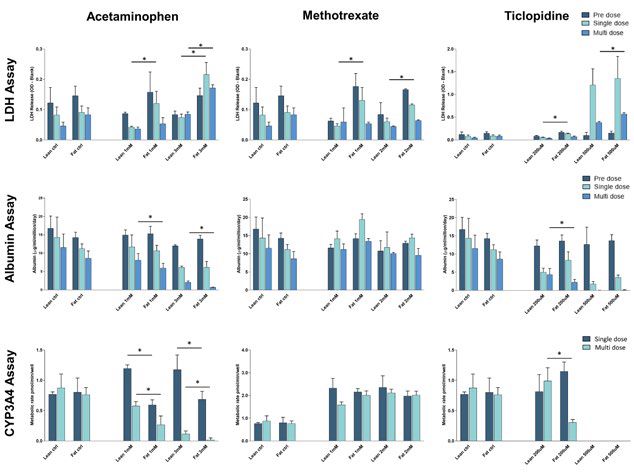
Figure 5. Fat loading of hepatocytes increases susceptibility to DILI by acetaminophen and ticlopidine. PHH were cultured for 14 days under fat conditions and then dosed with concentrations of compound around the IC:50 value previously determined. PHH microtissues were dosed with a single 48-hour dose or two 48-hour doses (multi-dose) of each compound. All compounds were dosed in lean media with a vehicle of 0.1 % DMSO. Control lean and fat microtissues were dosed with vehicle alone. Cell health was assessed by measuring LDH release in cell culture medium, albumin production in cell culture medium, and determining CYP3A4 activity of hepatocytes following dosing with the different compounds. Data are mean ± SD, n = 4. * = P < 0.05. Image Credit: CN Bio Innovations Limited
Conclusions
Using the PhysioMimix®, CN Bio generated a human in vitro model of MASLD. PHH were cultured in a fat-containing medium, which induced important features of early-stage clinical disease, such as intracellular fat loading, changes to the expression of key genes (including those involved with metabolism and insulin resistance) and increased albumin production.
To determine how the activities of CYP enzymes are controlled by intracellular lipid accumulation, CN Bio also investigated the metabolic activities of PHH after fat loading. Mimicking in vivo (clinical) observations, increases in a wide array of enzymes were reported, including CYP1A2, CYP2C9 (1.5-fold), CYP2C19 (2-fold), and CYP2E1 (2.5 fold) activity.2
A minor reduction in CYP3A4 activity after fat loading was also observed. Regarding their susceptibility to DILI, the impact of these metabolic changes on the liver cells was investigated by assessing the toxicity of Ticlopidine, Acetaminophen, and Methotrexate in the in vitro MASLD model.
Both Ticlopidine and Acetaminophen caused more extravagant DILI responses in fat-loaded cells, resulting in increased LDH release, reduced albumin production, and decreased CYP3A4 activity (as a measure of cell viability). When the compounds were dosed multiple times onto the hepatic tissues the changes were often significantly more prominent.
In the presence of the fat-loaded hepatocytes, dosing with Methotrexate did not appear to cause DILI. Combined, this data demonstrates how the MPS platform and associated MASLD model can capture the main features of early-stage human MASLD and deliver a varied response to DILI, which has been observed in clinical studies.
This method will be valuable for analyzing the toxicity profiles of novel compounds and how they behave, and cause DILI, in diverse patient subsets.
References and further reading
- Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Nat Med 2018;24:908-922.
- Fromenty B. Drug-induced liver injury in obesity. J Hepatol 2013;58:824-6.
- Kostrzewski T, Cornforth T, Snow SA, et al. World Journal of Gastroenterology 2017;23:204-215.
- Proctor WR, Foster AJ, Vogt J, et al. Arch Toxicol 2017;91:2849-2863.
About CN Bio
CN Bio is a leading organ-on-a-chip (OOC) company that offers a portfolio of products and contract research services to optimize the accuracy and efficiency of bringing new medicines to market. With more than a decade of research and development experience, they aim to transform the way human-relevant pre-clinical data is generated through the development of advanced in vitro human organ models.
CN-Bio's PhysioMimix® Core microphysiological system (MPS) enables researchers to recreate human biology in the lab and is the only microphysiological system with validated performance across single-, multi-organ, and higher throughput configurations. This easy to adopt, adapt and scale technology bridges the gap between traditional cell culture and human studies, to support the development of safer and more efficacious therapeutics, whilst reducing the dependence on animal model usage.
CN Bio’s portfolio of products (MPS, 3D validated cells, consumable plates) and services support researchers that require reliable, data-rich, in vitro studies, to uncover novel mechanistic insights into drug or disease mechanism of action.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.