For those considering adding Organ-on-a-chip (OOC) technology into the workflow, it may be unclear where to start.
Firstly, using a commercially available solution, such as CN Bio's PhysioMimix®, simplifies the transition from 2D, or more basic 3D organoid/spheroid assays.
From here, it is important to ensure that the data generated using the device is meaningful. This requires the 5Ps: Prior Planning and Pre-validation to ensure Perfect assay Performance.
During this phase, the right cells must be chosen. Some cells do not perform well in OOC assays and not all cell types provide the best degree of human relevance. This article explores the steps taken by CN Bio during the experimental planning phase to pre-validate cells for use in OOC assays.
Keep reading to identify the simplest way to invest in your success - choose 3D-validated primary cells from the CN Bio portfolio or embark on your own pre-validation studies.
Why use primary human cells over other cell types in OOC assays?
One of the most debated topics in the scientific community is choosing between a cell type, cell lines, primary cells, or induced pluripotent stem cells (iPSCs).
Engineered cell lines handle easily, are readily available and cost-effective. They also deliver precise and reliable data. This means that engineered cell lines are great for high throughput screening settings. However, they often lack key cellular components and exhibit cancer-like characteristics, limiting their ability to recapitulate human physiology. In terms of human translatability, their accuracy is limited.
IPSCs offer improved human relevance over engineered cell lines, making them a better match for OOC assays. iPSCs are artificially generated cells that have been reprogrammed from adult cells. They can differentiate into any cell type and can expand in culture.
However, iPSCs may not fully recapitulate the characteristics of mature primary human cells, and cannot offer the same level of genomic stability as primary human cells. They are also susceptible to spontaneously differentiating into other cell types.
Primary human cells are isolated directly from human tissue and have not undergone genetic modification or been transformed in any way. This means that primary human cells cannot be expanded in culture; however, when used in OOC assays, they offer a more accurate recapitulation of human in vivo response - particularly where multiple tissue-resident cells are combined.
Culturing primary cells under flow perfusion (to mimic the bloodstream) is game-changing for primary cell culture. For primary human hepatocytes (PHHs), flow perfusion extends cell longevity up to a month compared to just a few days in static culture, allowing enough time to induce disease or perform in vitro repeat dosing studies.
Comparability to in vivo responses can be displayed throughout by quantifying the activity of specific enzymes (such as the P450 family for PHH), or the release of cell-specific proteins into the culture medium.
Since the purpose of OOC, also known as microphysiological systems (MPS), is to recapitulate human physiology as accurately as possible, CN Bio deliberately chooses to use primary human cells when possible - although it is important to remember that any cell type can be used.
Handling primary human cells
Primary human cells have been on the receiving end of “bad press” due to their handling, retention of their native identity, and survival throughout an in vitro experiment. Historically, culturing and handling these cells was seen as a herculean task, because they have rather specific requirements.
Standard 2D static cultures utilizing plastic consumables have impaired the performance of these challenging cells, however, recent “ease of use” advances and the incorporation of flow perfusion by commercialized MPS/OOC platforms have made their culture much simpler and accessible for scientists without previous experience.
Optimal primary cell handling techniques differ between cell types, but the most important steps to perform correctly are the seeding and thawing procedures. As an example, PHHs represent a “diva-like” cell type. They demand specialized media throughout the thawing process, and slow pipetting and wide bore tips when seeding.
But do not let this be a discouragement, standard operating procedures from OOC vendors provide all the tips and tricks needed to succeed.
The importance of pre-validating primary human cells for OOC
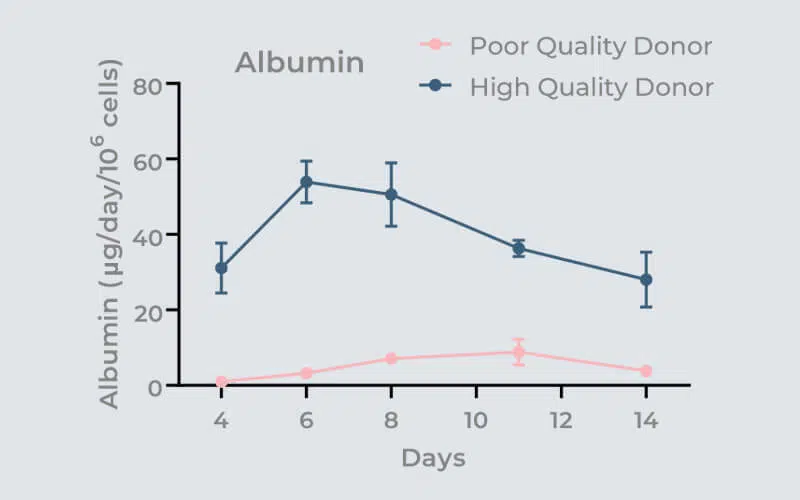
Image Credit: CN Bio Innovations Limited
Even when the thawing/seeding protocol has been followed to the letter, it does not guarantee that the cells will perform well in 3D OOC culture. The importance of pre-validating cells before OOC use is commonly overlooked and underestimated. The use of non-validated cells in OOC assays may result in variable assay performance, poor microtissue formation, and unreliable data.
Over the past five years over fifty primary human hepatocyte lots, from multiple cell suppliers, have been tested by CN Bio. Over 60 % failed to meet acceptable performance criteria. Most failed to even form 3D liver microtissues.
Calculations show that finding two donors that pass the validation procedures costs between $10,000 and $15,000 and can use over 100 hours of a scientist’s time - but this is time well spent. Utilizing pre-validated cells in OOC assays provides cross-experiment consistency and improved data robustness. But if this sounds like a lot of work, don't worry! It is possible to purchase pre-validated cells off the shelf!
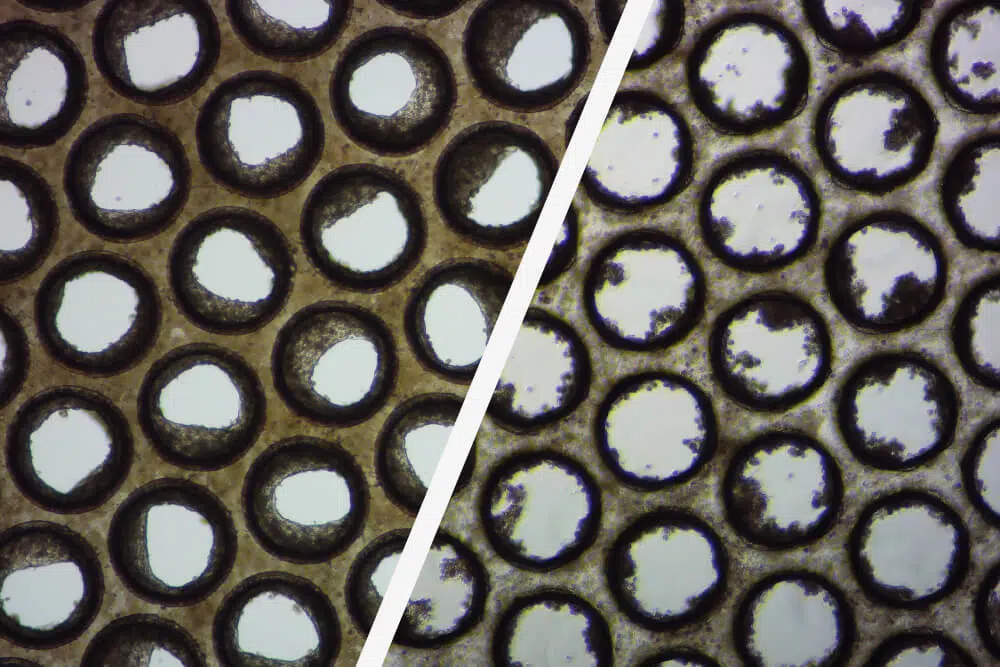
Image Credit: CN Bio Innovations Limited
Why does CN Bio recommend purchasing pre-validated cells?
Having partnered with providers of high-quality cells, including LifeNet Health LifeSciences and Lonza, CN Bio provides access to convenient, off-the-shelf, high-quality OOC-validated cells while avoiding the burden of in-house quality control experiments.
Its portfolio of 3D-validated cells allows users to successfully recreate advanced in vitro liver-on-a-chip models in their laboratory, which removes the risk of failed experiments caused by untested cells.
OOC-validated cells are slightly more expensive than the price of directly purchased cells. However, this reflects the comprehensive nature of the experimental work behind the scenes!
How to perform your own pre-validation studies
Although PhysioMimix® customers are encouraged to use validated cells from the catalog wherever possible, it is understood that some geographic regions are subject to import restrictions that negate this. The user may also wish to develop their own models from the ground up, own an alternative solution, or possibly have an internally developed platform. Don't be tempted to cut a corner and dive straight in is the advice from CN Bio, who describe how they validate primary human cells for OOC assays in the example below.
What are the most important considerations when performing in-house pre-validation assays on your own?
It is vital to remember that humans show intra-species variability. Differences in the population must be considered and can be embraced to study the different drug responses in patient cohorts - like the 25 % of our global population with fatty livers.
Depending on the study requirements, donor sex, age, ethnicity, and life exposures could be explored and carefully matched before donor selection. Outside of the inherent population-based variability, differing cell extraction methods between cell providers can introduce further variation as well.
To assess the variability in donor-to-donor performance, thorough testing is required using your choice of OOC platform versus the future context of use (or application). To begin, decide how many cell types are required to achieve your assay objectives. For instance, in drug metabolism studies, it is recommended to keep it simple with a PHH-only model.
For drug-induced liver injury assays, is it necessary to increase assay sensitivity by incorporating aspects of the body’s innate immune system? If so, include Kupffer cells (KC).
When modeling liver disease, decide which cell types contribute to inducing the disease’s phenotype and pathophysiology and factor these in. However, keep in mind that the cost and length of the validation process will increase as well.
Next, identify which cell health and functional markers are important to monitor for the selected cell types, as well as pass/fail thresholds. This information can be sourced from clinical data and earlier literature. Thresholds should be adjusted to reflect the reduced cell numbers used in the model versus the human organ.
What does an example tri-culture cell validation process look like?
CN Bio has developed a model of the human metabolic disorder non-alcoholic steatohepatitis (NASH), also known as Metabolic-dysfunction associated steatohepatitis (MASH) with the use of PHHs, hepatic stellate cells (HSC), and KCs. Below is an example of a tri-culture validation process using this model.
The first step is to pre-select a number (between 5 and 10) of PHH donors and identify the primary cell health and functional markers needed to compare each donor’s performance, including pass/fail thresholds.
To assess the tissue formation on scaffolds, it is recommended to perform tissue-specific immunofluorescent staining and subsequent confocal microscopy/analysis to confirm visually the quality of the 3D cultures. For PHHs, you can also quantify several functional biomarkers, including Urea, LDH, Albumin, and CYP3A4.
To guarantee the longevity of the liver tissue culture, assess the selected PHH donors for fourteen days using an OOC platform. Media samples should be collected every 2-3 days to assess whether donor cultures remain within, or outside, acceptable cell function and health limits at each time point. This process is typically run twice to look for the reproducibility of the donor’s characteristics.
The next step involves selecting several KC donors and testing for responses to an inflammatory stimulus, in this case, Lipopolysaccharides (LPS). To examine their response, inflammation markers, such as tumor necrosis factor-alpha (TNFα) and interleukine-6 (IL-6), are quantified.
This is conducted as a co-culture with one or two successful PHH donors for fourteen days, whilst simultaneously treating with LPS as a KC inducer. To guarantee that the performance of co-cultured cells stays within acceptable limits, PHH health and cell function are assessed, as well as KC inflammatory responses, at different time points.
Step three involves finding several HSC donors to co-culture with pre-validated PHHs and KCs. Set up a triple culture experiment to test several combinations of PHHs, HSCs, and KCs, ideally creating unique combinations of donors. During validation experiments, CN Bio cultures the cells in high-fat media (HEP-Fat) to induce a MASH phenotype. As with earlier stages, the experiment is cultured for fourteen days to ensure the longevity of the culture.
From there, HSC inducers are selected; for example, transforming growth factor beta (TGFβ), which specifically activates HSCs. Use these to treat the cells and determine whether the co-cultures provide a more advanced fibrotic phenotype once induced.
Chosen endpoint assays, with pre-determined pass/fail cut-offs, must include fibrosis markers, such as Fibronectin, and TIMP-1, as well as immunofluorescent staining for fat and HSC activation.
Triple cultures that deliver stable performance ( within the chosen criteria limits for each cell type) over the fourteen days are now validated for use.
The validation process is simple and logical for those with experience; however, the first step is the most crucial in the procedure. As previously stated, around 60 to 70 % of PHH donors will fail to culture in a 3D OOC environment. Additional cell types are far more likely to seed successfully when cultured using a good PHH donor.
In CN Bio’s experience, approximately 25 % of triple culture combinations fail. It is essential to budget tens of thousands of dollars and allow for a four to six-month testing period to run a similar study to this. However, for MASH, it is possible to circumvent the process should you so desire.
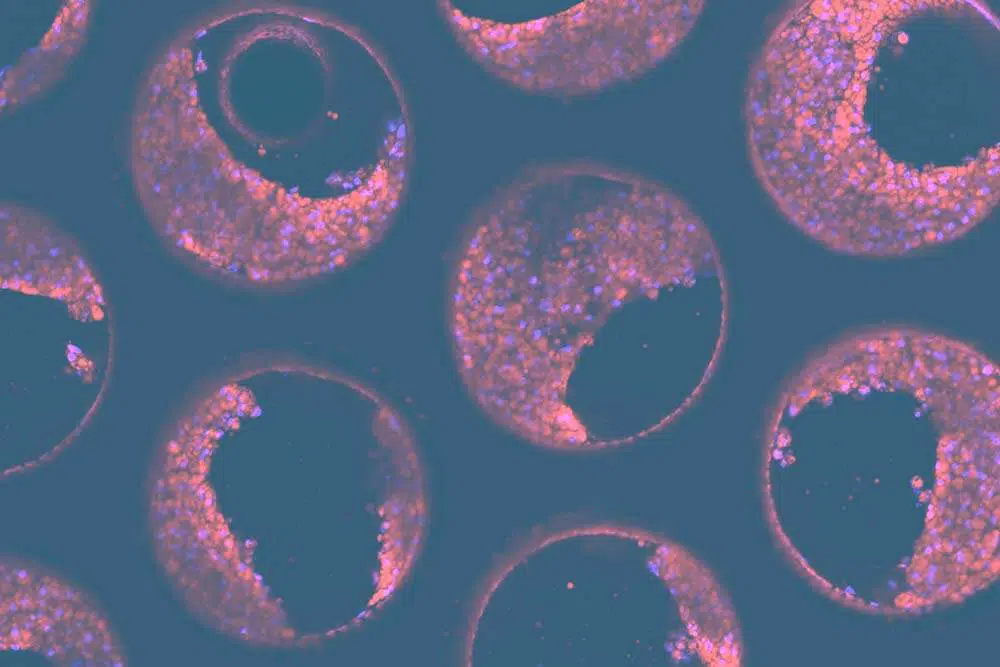
Image Credit: CN Bio Innovations Limited
NASH-in-a-box reagent kits
This validation principle has been applied to a unique product called NASH in-a-box, which is designed for use with the PhysioMimix® range of microphysiological systems.
This kit offers 3D validated triple cultures consisting of PHHs, KCs, and HSC, as well as thawing media, proprietary NASH-inducing cell culture media, quality control assay kits for liver microtissues, Multi-chip Liver-12+ consumable plates and supplements for cell culture.
Together with software-guided protocols (which offer a step-by-step walkthrough of the experimental process), the kit simplifies the whole workflow, allowing you to benefit from over a decade of experience. The kits also provide a choice: spend valuable time making new discoveries, or on validating cells.
What is next?
CN Bio customers have largely benefited from using its portfolio of 3D-validated liver cells, or NASH-in-a-box kit, to expedite OOC adoption in their workflows.
As CN Bio continues to diversify the range of models offered to include additional organs, it also works to develop a portfolio of 3D-validated cell and “in-a-box” kits.
About CN Bio
CN Bio is a leading organ-on-a-chip (OOC) company that offers a portfolio of products and contract research services to optimize the accuracy and efficiency of bringing new medicines to market. With more than a decade of research and development experience, they aim to transform the way human-relevant pre-clinical data is generated through the development of advanced in vitro human organ models.
CN-Bio's PhysioMimix® Core microphysiological system (MPS) enables researchers to recreate human biology in the lab and is the only microphysiological system with validated performance across single-, multi-organ, and higher throughput configurations. This easy to adopt, adapt and scale technology bridges the gap between traditional cell culture and human studies, to support the development of safer and more efficacious therapeutics, whilst reducing the dependence on animal model usage.
CN Bio’s portfolio of products (MPS, 3D validated cells, consumable plates) and services support researchers that require reliable, data-rich, in vitro studies, to uncover novel mechanistic insights into drug or disease mechanism of action.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.