The liver is accountable for metabolizing most medications and is therefore one of the primary tissues affected by adverse drug reactions. Most classes of medicine can cause drug-induced liver injury (DILI).
Unfortunately, DILI can pass undetected during pre-clinical drug development. It is also not uncommon for DILI to be first detected in the clinic, where it can stop trials while awaiting further investigations. Often there is little to worry about, as ceasing the medication usually resolves the DILI effect.
That said, it can be disconcerting to know that there is at least some degree of DILI risk in 750 FDA-approved drugs.1 There is also a long list of potential new drugs that have not been approved due to an excessive DILI risk.
The potential DILI liabilities in humans need to be more quickly identified in the pre-clinical discovery pipeline for attrition rates to decline. This would allow for promising drugs to return to the drawing board to “engineer out” potential toxicity issues.
However, there are numerous obstacles to overcome to de-risk this process efficiently. This includes:
- The limited relevance of animal DILI studies to humans
- Animal models have a history of incorrectly identifying toxic drugs as safe and labeling drugs as toxic when they are potentially useful.2
- Animal physiology is less suitable for testing new human-specific drug modalities.
- The complexity of the standard in vitro test models needs improvement to ensure that they behave and respond to drugs in a way that more closely represents the human body
- Historically, employing traditional methods to screen idiosyncratic responses, caused by genetic susceptibility or pre-existing liver disease, has been difficult.
The limiting simplicity of 2D liver cultures coupled with the inconsistencies of animal models has resulted in an immediate need for more predictive pre-clinical in vitro tools to investigate DILI.
There has been a recent focus on 3D cell culture platforms. Such methods produce functional and complex human in vitro liver models that have the potential to address these challenges and guide better-informed decision-making during drug development.3
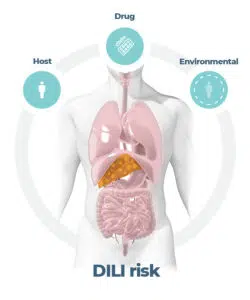
Figure 1. DILI is a multi-factorial pathogenesis. Image Credit: CN Bio Innovations Limited
The in vitro DILI testing workflow
Drugs must pass a large volume of tests in early discovery, beginning with basic high throughput batch screens for cell viability, which investigate whether the drug kills hepatocytes grown in 2D. In the preclinical stage of drug development, there are far fewer concentrations and drugs to test. This allows for the use of lower throughput, more in-depth 3D tissue models. Using this method, researchers can more accurately predict in vivo human DILI risk and its mechanism of action before entering the clinic.
Since the liver is a 3D tissue, 3D hepatocyte models, such as cell spheroids, exhibit improved results compared to 2D. Cells form physiologically relevant cellular interactions, resulting in more in vivo-like morphologies, extended culture longevity, and metabolic activity.
2D batch screens generally identify approximately one-third of drugs that have adverse effects on the liver. However, a collaborative study between AstraZeneca and InSphero, employing 3D primary human hepatocyte and non-parenchymal cell spheroids, improved upon this by detecting up to 60 % of DILI-positive compounds.4
However, a significant amount remains undetected. Are there advancements beyond 3D that can help detect all drugs with DILI risk?
Some spheroid methods can cause hepatocyte polarization; however, these models typically do not mimic the tissue-level structure seen in the liver. Hepatocytes are also exceedingly metabolic and need lots of oxygen delivery, which presents a challenge for spheroid cultures that infamously lack nutrient diffusion.
Without recapitulating high-order characteristics of the liver, toxic drugs with very specific or subtle actions may go unnoticed. There are also variables outside of the cell culture that can affect DILI.
Other tissues and organs can modulate how hepatocytes metabolize drugs. For example, the kinetics of drug absorption by the gut can influence metabolite-driven toxicity within the liver. Similarly, the presence of disease pathophysiology, such as Metabolic dysfunction associated liver disease (MASLD), can also affect how the liver reacts to a drug, with the potential to exacerbate injury from smaller doses.
With the opportunity to incorporate these compounding factors inside the pre-clinical toolbox, there is greater assurance that only drugs with a low risk of liver injury would progress to clinical trials.
Gaining more relevant information through ‘flow’
The aforementioned limitations can be addressed by integrating perfusion into 3D cultures. Using an Organ-on-a-chip (OOC) approach, the microarchitecture of liver tissue can be closely reproduced by growing physiologically relevant compilations of primary liver cells (including those with genetic variants that pre-dispose patients to disease) on continually perfused 3D scaffolds.
With fluid continuously circulating to provide polarization, mechanical stimulus, and consistent oxygen delivery, hepatocytes in these microenvironments maintain high functionality for up to four weeks.
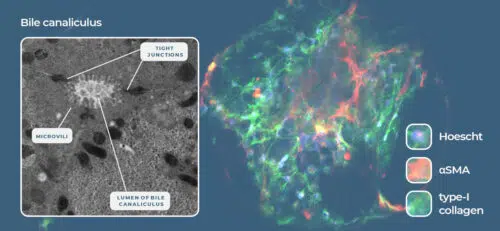
Figure 2. Liver-on-a-chip models recreate the liver microarchitecture and can recapitulate the phenotype of metabolic diseases such as NASH. Image Credit: CN Bio Innovations Limited
As well as expanding the possibilities of chronic dosing studies in vitro, these perfused OOC models offer the flexibility to uncover immune-related toxicity issues by incorporating Kupffer cells and circulating immune cells into the system. There is also the opportunity to induce common liver disorders, such as MASLD and Metabolic dysfunction associated Steatohepatitis (MASH), to uncover underlying morbidity susceptibilities.
To address the multi-organ effects on the toxicity of a compound, flow enables the linking of individual OOCs into multi-organ systems to simulate essential processes (like metabolism and drug absorption) and provides a better understanding of organ interactions (like multi-organ toxicity, metabolite-driven toxicity, or inflammation), which may not have been apparent otherwise.
Although their throughput can be lower, OOCs enable researchers to extend beyond the simple identification of acute and chronic liver toxicity, or the translation of in vitro results into a clinical setting. They also offer the chance to investigate the cause.
Answering more questions with multiple endpoints
Although basic 3D liver models successfully identify the presence or absence of a liver toxicity signal earlier than ever before in drug discovery, they are complemented by more complex perfused OOCs - which provide further detail.
Volume is required to achieve data richness and high assay sensitivity. Five hundred thousand primary cells perfused by large amounts of media (1-2 mL) are needed to create enough power to replicate phase 1 and 2 metabolism. This is particularly significant, as the non-human metabolic profile of standard animal and in vitro models is among the reasons that they are poor predictors of DILI.
A high volume of primary cells is also needed to increase assay sensitivity so that more sophisticated biomarker outputs of liver function, like ALT/AST, are detectable. Smart investment in these larger-scale OOC models pays dividends, as scientists can now discover unique insights that cannot be identified using simpler 2D or 3D in vitro systems, or even perfused Microphysiological Systems (MPS), which utilize microscale “chip” technology.
Samples taken over time in longitudinal OOC studies provide biochemical assessments that create a long-term picture. The large amount of tissue recovered at the end of the experiment can be investigated in additional genomics or proteomics studies to explore gene up- and down-regulation to establish a more complete toxicology profile.
This high content capability enables researchers to extend past the simple identification of acute or chronic liver toxicity, or the translation of results gained in vitro (using lab-grown organs) into a clinical setting. It enables the exploration of the mechanism behind the cause. 5
Understanding the cause enables solutions
Understanding the mechanism underlying any toxicity problem is important. This information enables the team to reconfigure, and potentially rescue a project from failure. The efficiency of this iterative process is significantly increased with a toxicity profile or mechanistic fingerprint to inform the redesign. Biological insights are also helpful for determining what data is most important.
Occasionally, liver toxicology signals appear in pre-clinical animal testing that contradict clean human assays, or data from other in vivo animal species.
The generation of comparative human data before the first-in-human study provides confidence to progress with the confidence that the signal is probably a function of the test animal species, rather than the compound itself. This knowledge can prevent a project from being unnecessarily terminated.
Gaining toxicology insights beyond small molecules
Conventional toxicology research has focused on small-molecule compounds. However, novel human-specific modalities (such as products encapsulated in lipid nanoparticles, biologics, or oligonucleotide/RNA-based therapies) have moved to the forefront of discovery and development.
While they are less prone to DILI, these new products are still capable of inducing liver injury. An example of this is the Astellas-Audentes gene therapy trial for myotubular myopathy. This therapy seemed to offer promising results; however, liver failure caused the death of four patients and called the future of the project into question.
In scenarios like these, OOC is particularly beneficial for assessing safety, as it removes the roadblocks of metabolism, genetics, and/or immunological responses, which can render animal models unviable, while also addressing the lack of architecture and physiological flow of in vitro platforms. OOCs are ‘agnostic’ to drug type and ensure the relevant receptors in the relevant parts of the tissue take up relevant molecules.
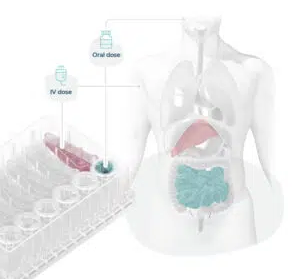
Figure 3. Organ-on-a-chips mimic organ phenotypes, enabling scientists to recreate human-relevant drug dosing and associated toxicity. Image Credit: CN Bio Innovations Limited
Range of approaches
OOC allows in-depth toxicological insights that were not previously possible. Keen regulatory interest and political pressure to reduce animal usage in drug discovery, and an established consensus about OOC’s effectiveness, are driving its adoption. If OOC is embedded in drug discovery to uncover safety toxicology issues earlier, there will undoubtedly be reduced risk and increased efficiency.
Biotech and pharmaceutical companies are exploring OOC technologies. Bigger companies may benefit from establishing in-house protocols while smaller companies, or virtual biotech firms, may find that full-service expert support offers a more practical approach.
It is also important to note the distinction between different OOC systems: they are similar in some ways, but drastically different in others. This means it is crucial to pick the right OOC for the task.
A system that is simple to operate, provides high yields of biological materials for sampling, incorporates physiologically relevant flow, and is ‘agnostic’ to therapy modality is well suited for identifying and characterizing DILI.
CN Bio offers various OOC options, including an in-house adoption (PhysioMimix system), and a complete Drug Metabolism and Safety Toxicity Testing Service. The latter assists with determining the mechanistic aspects of drug-induced liver toxicity in comprehensive detail for those looking to outsource.
CN Bio’s systems use more traditional lidded multi-chip plates (12- or 48-chips/plate) that will be familiar to most cell culture scientists. They provide large amounts of recoverable sample (tissue and media) while maintaining continuous flow to generate physiologically relevant results.
References and further reading
-
Thakkar, S., et al. (2018). The Liver Toxicity Knowledge Base (LKTB) and drug-induced liver injury (DILI) classification for assessment of human liver injury. Expert Rev Gastroenterol Hepatol. doi.org/10.1080/17474124.2018.1383154.
-
Van Norman, GA. (2019). Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl Sci. doi.org/10.1016/j.jacbts.2019.10.008.
-
Weaver, RJ., et al. (2020). Managing the challenge of drug-induced liver injury: a roadmap for the development and deployment of preclinical predictive models. Nat Rev Drug Discov. doi.org/10.1038/s41573-019-0048-x.
-
Proctor, WR., et al. (2017). Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury. Arch Toxicol. doi.org/10.1007/s00204-017-2002-1.
- Novac, O., et al. (2021). Human liver microphysiological system for predicting the drug-induced liver toxicity of differing drug modalities. [Online] CN Bio. Available at: https://cn-bio.com/resource/human-liver-microphysiological-system-for-predicting-the-drug-induced-liver-toxicity-of-differing-drug-modalities/.
About CN Bio
CN Bio is a leading organ-on-a-chip (OOC) company that offers a portfolio of products and contract research services to optimize the accuracy and efficiency of bringing new medicines to market. With more than a decade of research and development experience, they aim to transform the way human-relevant pre-clinical data is generated through the development of advanced in vitro human organ models.
CN-Bio's PhysioMimix® Core microphysiological system (MPS) enables researchers to recreate human biology in the lab and is the only microphysiological system with validated performance across single-, multi-organ, and higher throughput configurations. This easy to adopt, adapt and scale technology bridges the gap between traditional cell culture and human studies, to support the development of safer and more efficacious therapeutics, whilst reducing the dependence on animal model usage.
CN Bio’s portfolio of products (MPS, 3D validated cells, consumable plates) and services support researchers that require reliable, data-rich, in vitro studies, to uncover novel mechanistic insights into drug or disease mechanism of action.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.