Absorption, distribution, metabolism, and excretion (ADME) are four main processes that demonstrate how a drug behaves after being administered. Therefore, ADME is key in defining a compound’s pharmacokinetic (PK) properties and bioavailability.
Oral bioavailability is determined by how much of the drug reaches systemic circulation following absorption across the intestinal wall and first-pass metabolism in the liver. ADME and bioavailability are vital constituents for identifying how the body processes drugs and their toxicology profiles. These properties are determined during the preclinical stages of drug discovery and development to inform dose-setting in the clinic.
To profile oral bioavailability using current methods, a combination of simple in vitro assays that model either the gut (Caco-2 cell line) or the liver (liver microsomes and suspension hepatocytes) and in vivo animal models are typically used. However, both approaches are extremely limited in methodology.
Caco-2 cells, long considered the benchmark for assessing in vitro intestinal permeability, cannot account for how the liver metabolizes. Moreover, the cell line has low levels or lacks enzyme and transporter expression. Liver microsomes and suspension hepatocytes are typically employed for in vitro drug metabolism screening studies but do not account for intestinal absorption. Together, these approaches are holding back the true accuracy of their estimations.
In a formative study investigating 184 compounds, animal models demonstrated a weak correlation with human bioavailability (R2=0.34).1 Therefore, developing a new human-oriented approach that combines oral absorption and hepatic metabolism is necessary to estimate drug bioavailability with improved accuracy.
Over the last decade, microphysiological systems (MPS), also known as organ-on-a-chip (OOC), have shown promise to improve the human translatability of ADME studies. They have been developed to more accurately recapitulate the structural and functional biomarkers of cells and tissues in vitro.
Efforts to boost the in vitro to in vivo translation of ADME data have resulted in the development of a more intricate MPS where several organs, such as gut and liver, are linked together fluidically to replicate processes such as drug absorption and first pass metabolism.2
Aim
By developing a dual-organ MPS that interconnects a standard primary human liver model with a primary human intestine model, CN Bio aims to improve the in vitro estimation of human bioavailability.
To connect the primary gut tissues with the liver tissues, CN Bio has developed a chemically defined media to support the culture of both organ models. This media facilitates the maintenance of hepatic metabolic functionality and intestinal barrier integrity.
Leveraging the credibility of well-established drug compounds, CN Bio intends to demonstrate the enhanced predictive capabilities of its primary Gut/Liver MPS for profiling the ADME behavior of oral drugs compared to an equivalent Caco-2 Gut/Liver MPS.
Methods
This study combined a dual-organ MPS that links a primary human intestine (RepliGut® Planar-Jejunum, Altis Biosystems) with a primary human Liver-on-a-chip (CN Bio). The dual-organ MPS is cultivated using the PhysioMimix® system and its custom-made “Multi-chip” Dual-organ consumable plate, as shown in Figure 1A.
The Dual-organ plate comprises six wells, each incorporating two compartments: (i) a Transwell® compartment and (ii) a liver compartment. It is possible to control fluidic flow independently in each compartment (intestine and liver) and the adjoining channel between the organs, as demonstrated in Figure 4A.
The intestinal barrier was replicated by expanding human jejunum stem/progenitor cells across a Transwell® coated with a biomimetic scaffold. This was followed by cell differentiation into a polarized barrier of post-mitotic lineages found in the human intestine.
The media was exchanged with either RepliGut® Growth or Maturation media every 48 hours. During the same period, intestinal barrier integrity was evaluated by measuring transepithelial electrical resistance (TEER) or establishing the permeability of Lucifer yellow across the Transwell once the dual-organ experiment had concluded.
The expression of markers that verified intestinal origin (Villin and CDX2) and the presence of the tight-junction marker ZO-1 were substantiated through fluorescent microscopy. To validate mucus production, histological staining of the RepliGut® Planar-Jejunum cross-section with Alcian blue was performed alongside in-well immunofluorescent staining for the Muc-2 protein.
CN Bio used RNA isolated on day 15 of Caco-2 culture, or on day seven post-differentiation of RepliGut® Planar-jejunum, to assess the expression of metabolic and transporter genes. TaqManTM Gene Expression Assays were used to measure qPCR of the gene expressions of metabolic enzymes and transporters, while relative expression was determined via ΔΔCT analysis.
For the liver, primary human hepatocytes (PHH) were seeded in the liver compartment of the PhysioMimix on a perfused 3D collagen-coated scaffold (Figure 1C). On day four (after PHH seeding and microtissues had formed), differentiated RepliGut cultures were introduced to the PhysioMimix to establish the primary Gut/Liver co-culture.
A chemically defined media was used to preserve and maintain gut and liver tissue functionality for at least 48 hours, while compounds were introduced to evaluate their ADME properties. During this study, the performance of the primary RepliGut/liver MPS was compared to that of a Caco-2 Gut/liver MPS.
To establish the Caco-2 Gut/liver MPS, Transwells with differentiated Caco-2 monolayers (at 15-17 days post-seeding) were inserted into the PhysioMimix on day four, following PHH seeding and the formation of microtissues.
7-hydroxycoumarin (7-HC), a fluorescent compound that undergoes Phase II metabolism by glucuronidation, was used in a proof-of-concept study to establish absorption through the intestinal barrier and subsequent first-pass metabolism in the co-culture model.
The PhysioMimix enables a flexible compound dosing technique with either Gut/Liver in co-culture or gut only (no PHH) and liver only (no gut barrier, with compounds dosed into a blank Transwell with no cells). The model was challenged by two compounds whose human ADME properties were not predicted by traditional preclinical approaches (Temocapril and Midazolam).
On day four after the seeding of PHH and the addition of the gut tissues to Dual-organ plates, Temocapril and Midazolam were introduced by either oral (drug introduced to the apical surface of the Transwell) or intravenous (IV) dosing (liver only, drug mixed into co-culture media) dosing.
Media samples were taken at intervals of 0, 1, 4, 6, 24, and 48 hours and evaluated using liquid chromatography-mass spectrometry (LC-MS) to establish the concentration of parent compounds in the liver compartment.
An area under the curve (AUC) estimation of both oral and IV concentration profiles was generated using GraphPad Prism.
Results and discussion
The PhysioMimix (Figure 1) revealed that a primary Gut/Liver MPS could overcome the human-relevance limitations associated with current models for profiling oral drug bioavailability.
In the RepliGut model (Figure 2A), jejunum stem and progenitor cells were extended to confluence on a biomimetic scaffold before undergoing differentiation. This method, as shown in Figure 2B, increased barrier strength.
Gene expression demonstrated that proliferative cell genes were downregulated while differentiated enterocyte genes were upregulated relative to the cells in the proliferative phase (Figure 2B). In the differentiation phase, the RepliGut model expressed tissue-specific markers that confirmed intestinal origin, in addition to markers of tight junctions and mucins, verifying multi-cellular lineages (Figure 2C).
This was confirmed using histology in combination with observations of a distinct and continuous layer of mucus at day seven post-differentiation. In contrast, mucus production was absent in the epithelial cell line, Caco-2 model (Figure 2D).
To evaluate the potential of the RepliGut model’s ability to enhance predictivity over the Caco-2 model, the expression of major metabolic enzymes and transporter genes (key elements in drug metabolism and active drug transport), was investigated. Compared to the Caco-2 cell line, there was an improvement in gene expression (Figure 2E).
Establishing the conditions that maintain the functionality of both tissue types presents a significant challenge in the co-culture of two or more tissues. CN Bio has developed a chemically defined media that is introduced to the Transwell basolateral and liver compartments of the Dual-organ plate (Figure 3A).
This media helped maintain hepatic cell health and functionality in co-culture for a minimum of 48 hours, measured by LDH release, CYP3A4 activity, and albumin production (Figure 3 B-D).
Moreover, this media preserved the integrity of the intestinal barrier over the 48 hours of co-culture, as measured by TEER (Figure 3E) and a Lucifer yellow permeability assay (Figure 3F), after introducing the RepliGut model to the dual-organ plate.
Functionality was confirmed by 7-HC dosing, as shown in Figure 4A, which experiences metabolism by way of glucuronidation (Figure 4B), with both intestinal and hepatic tissues aiding its clearance (Figure 4C).
To demonstrate the enhanced capacity of the primary RepliGut/ Liver MPS compared to a Caco-2 Gut/Liver MPS, CN Bio assessed the properties of two drugs whose bioavailability had been poorly predicted by traditional approaches.
Temocapril was the first drug tested, a pro-drug developed to resist intestinal hydrolysis that is metabolized to Temocaprilat by carboxylesterase 1 (CES1) (Figure 5A). The isoenzyme pattern in humans has been shown to express CES1 and CES2 within the liver and intestine, respectively.3
In Caco-2 cells, CES1 is primarily expressed, which leads to a mismatch that results in an overestimation of drug clearance (Figure 5B-C). Conversely, the more human-representative ratios of both CES1 and CES2 expression by the RepliGut model mean it is better suited to pro-drug studies (Figure 5G).
Leveraging oral and IV dosing methods, CN Bio was able to profile the clearance of Temocapril (Figure 5E). In the liver, rapid clearance was observed within 24 hours after administering an IV dose (Figure 5F). In accordance with intestinal CES expression, the primary RepliGut/Liver MPS was able to accurately report resistance to intestinal hydrolysis of Temocapril, which means less Temopcarilat was produced at 48 hours in the gut apical compartment after administering the drug orally.
Finally, CN Bio reviewed the bioavailability of Midazolam in both Gut/Liver MPS models. This drug is recognized for its ability to largely undergo intestinal clearance by the CYP3A4 enzyme.4 Midazolam was rapidly cleared by the liver following IV dosing, demonstrating its high inherent hepatic clearance rate (19 mL/min/Kg) (Figure 6A).
The primary RepliGut/Liver MPS cleared more midazolam than the Caco-2 Gut/Liver MPS (Figure 6B). It also delivered an oral bioavailability estimation that was more representative of human clinical observations (Figure 6C).
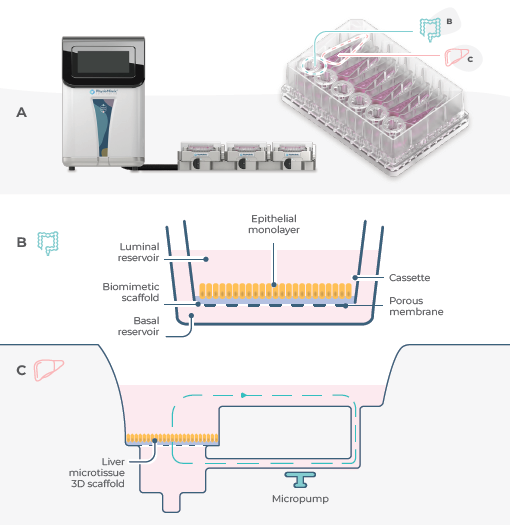
Figure 1. Set-up of a standard Gut/Liver MPS in the PhysioMimix. Image Credit: CN Bio
A) Assembly of the PhysioMimix System set-up and its Multi-chip Dual- organ plate, revealing the location of the gut and liver compartments within the plate. Each plate can culture up to six Gut/Liver models.
B) Schematic diagram of a RepliGut® model cultured on a biomimetic scaffold within a Transwell insert. This produces an intestinal epithelial barrier.
C) Schematic overview of fluidic flow within the liver compartment of the Dual-organ plate.
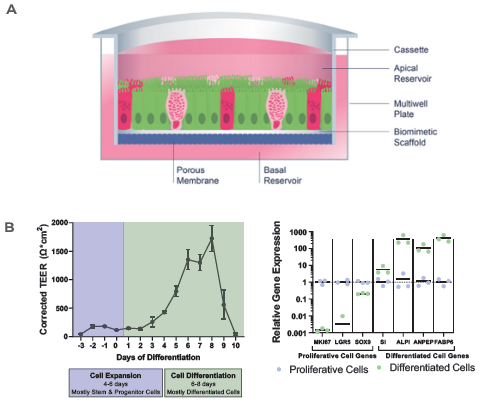
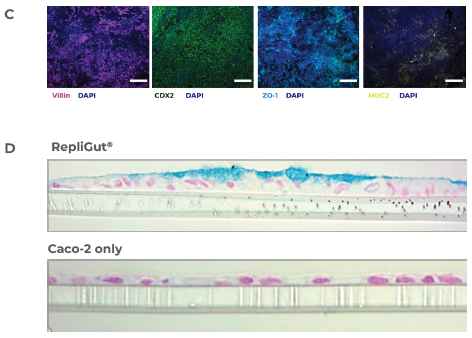
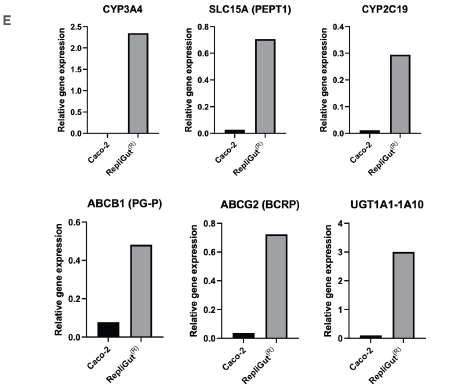
Figure 2. RepliGut's primary intestine model is more human-relevant than the standard Caco-2 cell line model. Image Credit: CN Bio
A) Crypt epithelium stem/progenitor cells are isolated from jejunum samples and extended across a biomimetic scaffold in static conditions.
B) TEER and relative gene expression measurements reveal barrier integrity in the RepliGut model's expansion and differentiation stages.
C) Immunofluorescence images of the RepliGut model: The barrier is stained for DAPI (dark blue), the tight junction protein, ZO-1 (light blue), and other intestinal markers, including MUC2 (yellow), violin (purple), and CDX2 (green). The image scale bars correspond to 100 μm for CDX2 and 200 μm for the other three markers.
D) Histology cross-sections of the RepliGut and Caco-2 models. Hematoxylin and eosin stain monolayer cross-sections to visualize nuclei and with Alcian blue to visualize the mucus layer.
E) qPCR as an example of relative gene expressions of essential transporters and metabolic enzymes in the RepliGut and Caco-2 models.
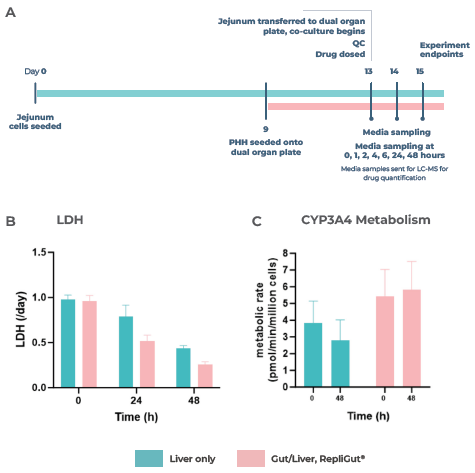
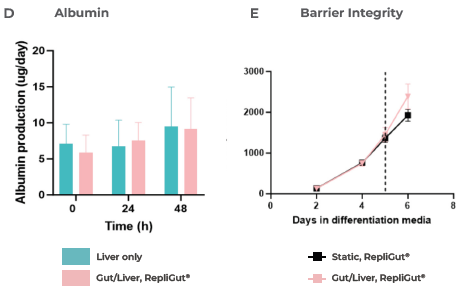
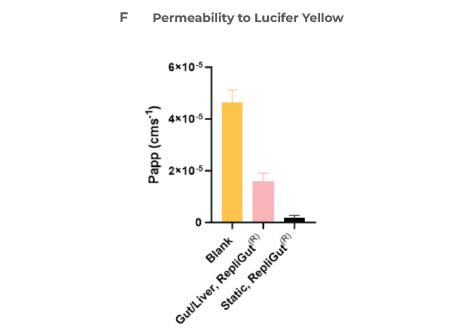
Figure 3. Liver and gut functionality markers are maintained throughout the primary cell Gut/Liver co-culture. Image Credit: CN Bio
A) Experiment timeline to determine the primary RepliGut/Liver MPS. RepliGut was initially cultured in static for 13 days before being transferred to the Transwell compartment of the PhysioMimix four days after PHH seeding.
A chemically defined media was used to maintain the co-culture for 48 hours. The liver cell health and functionality markers were measured at 0, 24, and 48 hours of co-culture, with a liver-only MPS cultured in parallel as a control.
CN Bio investigated B) LDH release, C) CYP3A4 activity, and D) Albumin production. The barrier integrity of RepliGut in the Gut/Liver MPS was evaluated by E) TEER and F) permeability to Lucifer yellow and compared against independent RepliGut cultured in static for the complete duration in standard RepliGut® Maturation media.
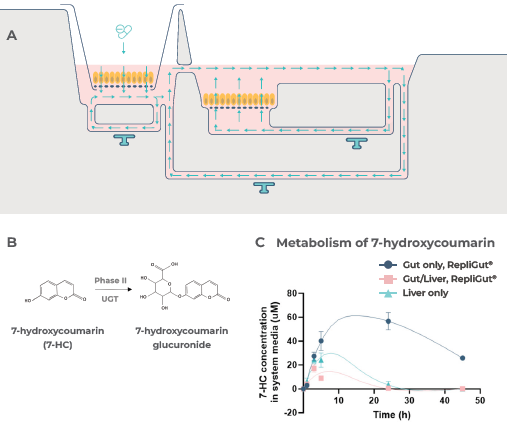
Figure 4. First-pass metabolism can be modeled by the primary cell Gut/Liver MPS. Image Credit: CN Bio
A) Schematic outline of 7-HC dosing in the Gut/Liver MPS model. 1mM 7-HC was dosed in the apical side of the Transwell compartment, which contained the RepliGut barrier within the Dual-organ plate.
Subsequently, 7-HC was transported across the barrier into the media in circulation between the gut and liver tissues. The RepliGut model was inserted into the Dual-organ plate Transwell compartment attached to a blank liver compartment (without PHH) to prepare the gut-only control.
Similarly, the liver-only control was prepared by dosing 7-HC into a blank Transwell insert (without gut cells) in the Transwell compartment of the Dual-organ plate.
B) Pathway of 7-HC metabolism. 7-HC experiences phase II metabolism by UGT enzymes to create the non-fluorescent metabolite 7-hydroxycoumarin glucuronide.
C) Demonstrates the changes in 7-HC concentration in the circulating media over time.
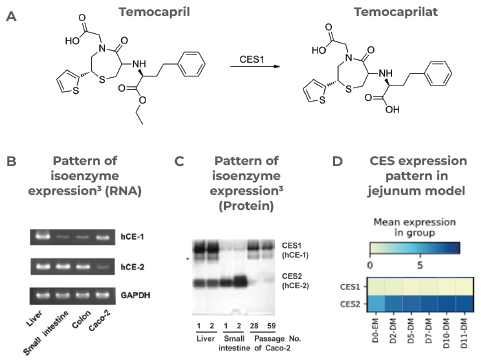
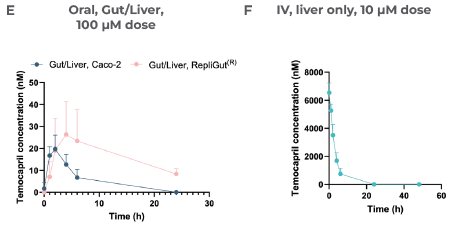
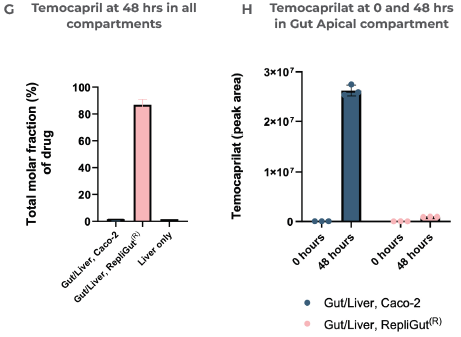
Figure 5. Case study 1, Temocapril. Resistance of Temocapril to intestinal clearance observed in the primary cell Gut/Liver MPS, correlated with isoenzyme expression in the human intestine. Image Credit: CN Bio
Temocapril was administered as either an oral dose (100 µM) in the apical side of the Transwell or as an IV dose (10 µM) and mixed directly with circulating media in the liver compartment.
Two Gut/Liver MPS configurations were evaluated: Caco-2/Liver and RepliGut/Liver. A) shows that Temocapril is predominantly metabolized by carboxylesterase (CES) 1 isoenzyme into the active metabolite Temocaprilat.
The pattern of CES1 isoenzyme RNA is shown in B) and Protein expressions in the human liver, small intestine, and the Caco-2 cell line C).3
The Pattern of CES gene expression in the Jejunum model over time in expansion media (EM) and differentiation media (DM) is shown in D). Scott Magness provided data at UNC Chapel Hill.
The concentration of Temocapril in the circulating media over time in the Gut/Liver models can be seen in E) and is measured by LC-MS.
Section F) reveals the Concentration of Temocapril over time in the liver-only model, while G) demonstrates the total molar fraction of Temocapril that remained across all compartments in each model at 48 hours. The relative amount of the metabolite Temocaprilat in the gut apical compartment in both Gut/Liver models at 0 and 48 hours after drug dosing is shown in H). Peak area is a qualitative measure of compound concentration.
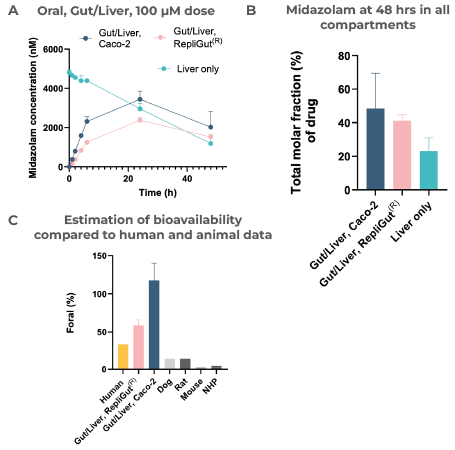
Figure 6. Case study 2, Midazolam. Improved correlation with human bioavailability of Midazolam by the primary cell Gut/Liver MPS. Image Credit: CN Bio
Midazolam was administered as either an oral dose (50 µM) in the apical side of the Transwell, within the gut compartment, or as an IV dose (5 µM) mixed directly with circulating media in the liver compartment. Two Gut/Liver MPS configurations were evaluated: Caco-2/Liver and RepliGut/Liver.
Over 48 hours, media samples were collected and quantified by LC-MS to determine A) the concentration of Midazolam in the circulating media following various dosing regimens and B) the total molar fraction of Midazolam that remained across all compartments in each model after 48 hours.
Moreover, as shown in section C, CN Bio was able to estimate oral bioavailability with both Gut/Liver MPS models by taking the ratio of the area under the curves and dose and comparing it with human and animal data in the published literature.1
Conclusion
This study revealed that the primary RepliGut/Liver MPS could more closely replicate the physiological conditions of oral drug dosing in humans. Combining intestinal absorption and hepatic metabolism, the primary RepliGut/Liver MPS creates in vivo-like drug concentrations that cannot be reproduced using conventional preclinical in vitro models.
The primary RepliGut/Liver MPS presents an improved method to assess the pharmacokinetics of pro-drugs that encounter CES metabolism in contrast to a comparable Caco-2 Gut/Liver MPS, which could not profile human bioavailability. The results reveal that the primary MPS model provides a practical alternative to tackle limitations associated with the Caco-2 cell line for this drug type.
When more human-relevant data is produced earlier in drug discovery, issues can be observed and flagged to address them before costly preclinical in vivo studies are conducted.
As the primary RepliGut/Liver MPS comprises primary human cells, there are no interspecies differences to consider. Therefore, the model can circumvent the poor correlation between animal model ADME predictions and human outcomes.
Closing the gap between in vitro assays and in vivo studies, the model allows researchers to identify and advance only the drug candidates that show the most promise with confidence, facilitating a wave of cost reductions while reducing the number of animal models required.
References and further reading
- Musther, H., Olivares-Morales, A., Hatley, O. J. D., Liu, B. & Hodjegan, A. R. Animal versus human oral drug bioavailability: Do they correlate? European Journal of Pharmaceutical Sciences 57, 280 (2014).
- Edington, C. D. et al. Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies. Sci Rep 8, (2018).
- Imai, T., Imoto, M., Sakamoto, H. & Hashimoto, M. Identification of esterases expressed in Caco-2 cells and effects of their hydrolyzing activity in predicting human intestinal absorption. Drug Metab Dispos 33, 1185–1190 (2005).
- Paine, M. F. et al. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther 60, 14–24 (1996).
About CN Bio
CN Bio is a leading organ-on-a-chip (OOC) company that offers a portfolio of products and contract research services to optimize the accuracy and efficiency of bringing new medicines to market. With more than a decade of research and development experience, they aim to transform the way human-relevant pre-clinical data is generated through the development of advanced in vitro human organ models.
CN-Bio's PhysioMimix® Core microphysiological system (MPS) enables researchers to recreate human biology in the lab and is the only microphysiological system with validated performance across single-, multi-organ, and higher throughput configurations. This easy to adopt, adapt and scale technology bridges the gap between traditional cell culture and human studies, to support the development of safer and more efficacious therapeutics, whilst reducing the dependence on animal model usage.
CN Bio’s portfolio of products (MPS, 3D validated cells, consumable plates) and services support researchers that require reliable, data-rich, in vitro studies, to uncover novel mechanistic insights into drug or disease mechanism of action.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.