Comprehending the thermal, chemical, and mechanical stability and physical characteristics of polymers is key to development success. The following case studies summarize experimental methods and data analysis techniques that ARGEN™ uses to determine molecular weight and how temperature, shear stress, and solution conditions affect the stability of an undefined organic polymer (PS-A) and Bovine Serum Albumin (BSA). This data is pivotal for accelerating formulation development.
Image Credit: Raimundo79/Shutterstock.com
ARGEN™: Smart and rapid therapeutic biopolymer development
ARGEN™ is a highly efficient instrument for rapidly assessing the stability and viability of therapeutic proteins, peptides, and biopolymers. The tool features a multi-stressor testing platform that works via static light scattering detection and intuitive data processing. These characteristics allow researchers to engineer biologic formulations up to 16 times more quickly.
How ARGEN™ works
ARGEN™ uses fixed-angle (90°) simultaneous multiple-sample light scattering (SMSLS) technology, which enables rapid, instantaneous, continuous data collection to determine the target molecule's qualitative and quantitative characteristics.
The device has 16 independently controlled sample cells, allowing users to simultaneously set parameters for thermal, chemical, and mechanical (stirring) stress on each sample. This enables a highly versatile approach to experimental design.

Image Credit: Yokogawa Fluence Analytics
ARGEN™ intuitive control software
The ARGEN™ control software has an easy-to-use interface for all experimental design factors and independent control of every cell for adjusting parallel parameters and instantaneous data processing.
Experimental methods
Polymer characterization
Two polystyrene (PS) standards at 16,500 g/mol and 185,000 g/mol and an undefined PS sample (PS-A) were continually diluted with tetrahydrofuran (THF) from 15 mg/mL to 0.166 mg/mL, resulting in an 8-point Debye plot for extrapolation of each sample’s absolute molecular weight via the Debye equation:
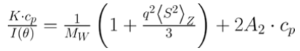
Monitoring polymer degradation
Polystyrene (PS-A) samples were dissolved in THF at a [PS-A] = 10 mg/ml, put in cuvettes, and added to ARGEN™ sample cells. Next, the samples were thermally stressed at a temperature ranging between 30 °C and 55 °C for 15 hours to track degradation.
Another experiment was carried out by subjecting samples to stirring (mechanical stress) at 100 RPM and thermal stress from 30 °C to 55 °C throughout the experiment.
Eventually, three samples of PS-A were dissolved in toluene (Tol), butyl acetate (BAc), or THF and left to degrade at 55 °C with a stirring rate of 100 RPM for 10 hours.
Monitoring biopolymer (protein) degradation
Utilizing Bovine Serum Albumin (BSA) as a model protein for aggregation and degradation, a [BSA] = 2 mg/mL sample was prepared in 50 mM phosphate. The pH of each solution was modified to a pH range of 1.54-5.85 (5 data points) with a final [BSA] of 1 mg/ml. The samples were then heated to 37 °C for five days utilizing ARGEN™’s temperature control feature.
Data interpretation and analysis
Polymer characterization
Before data collection, light scattering intensity was noted for each dilution. Each dilution was examined and tracked for at least three minutes to produce a stable baseline signal (Figure 1—inset).
![Debye analysis of a 16,500 g/mol polystyrene standard in THF. The data is fit using a linear least squares regression. The y-intercept yields the reciprocal of the molecular weight. [Inset] Baseline scattering intensities](https://www.news-medical.net/images/appnotes/ImageForAppNote_5272_17261258073918508.png)
Figure 1. Debye analysis of a 16,500 g/mol polystyrene standard in THF. The data is fit using a linear least squares regression. The y-intercept yields the reciprocal of the molecular weight. [Inset] Baseline scattering intensities. Image Credit: Yokogawa Fluence Analytics
Debye analysis was utilized to calculate the starting molecular weight of all samples, and multiple assumptions were required. If q2(S2) Z << 1 (which is the case for the majority of polymers) and the sample is diluted, then the equation can be simplified to become:
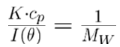
If reorganized for single angle analysis at 90° and solving for weight-averaged molecular weight, the equation changes to:
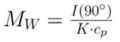
Via this equation and a linear fit of the dilution data (Figure 1), the 16,500 g/mol and 18,500 g/mol standards were determined to have molecular weights of 15,300 g/mol and 193,000 g/mol. The unknown PS sample had a molecular weight of 5500 g/mol (Figure 2).
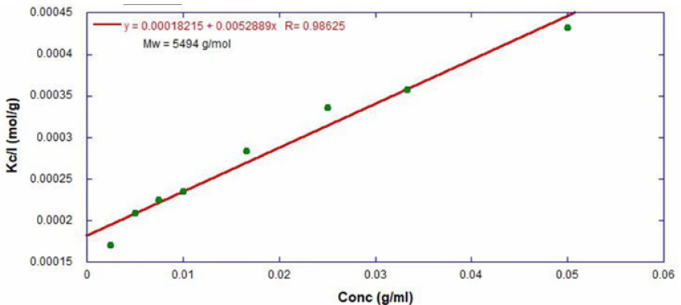
Figure 2. Debye analysis of a polystyrene sample with unknown molecular weight. The molecular weight was determined to be 5,500 g/mol. Image Credit: Yokogawa Fluence Analytics
Polymer degradation
The degradation curves were normalized to the polymer's initial mass, and the normalized molecular weight was plotted against time.
Without mechanical stress through stirring (Figure 3), the polymer was soluble at all temperatures and underwent no apparent change in molecular weight or indication of degradation.
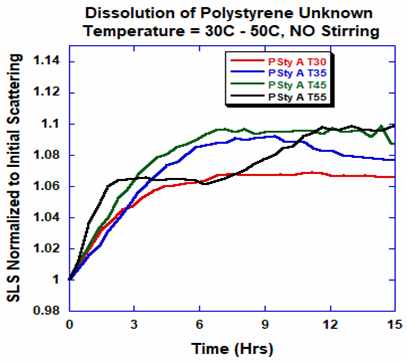
Figure 3. Dissolution profiles for PS-A at different temperatures with no stirring. Image Credit: Yokogawa Fluence Analytics
When subjected to mechanical (stirring) and temperature (heat) stresses, all of the samples were soluble within two minutes and degradation was seen instantaneously as suggested by the altered light scattering signal.
Samples under temperatures ranging from 30 °C to 45 °C demonstrated a comparable degradation profile (Figure 4).
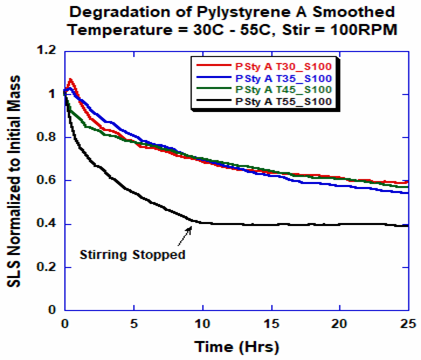
Figure 4. Degradation profiles for PS-A at different temperatures with stirring at 100 RPM. Image Credit: Yokogawa Fluence Analytics
The polymer dissolved rapidly at temperatures exceeding 55 °C (Figure 3).
As earlier discussed, removing mechanical stress or stirring stops degradation, as demonstrated by changes in light scattering intensity. Moreover, increases in degradation rate seen in temperatures ranging 45 °C to 55 °C indicate a considerable thermodynamic barrier linked to the depolymerization of polystyrene (Figure 5).
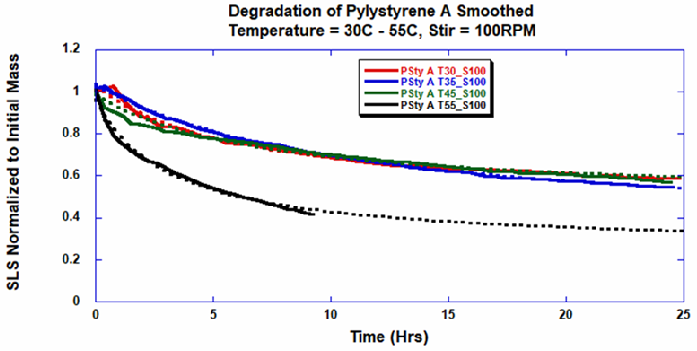
Figure 5. Polystyrene-A degradation at different temperatures with stirring at 100 RPM was fitted with a double exponential. Image Credit: Yokogawa Fluence Analytics
Solvent effects on polymer stability and solubility
THF and toluene demonstrated diverse effects on PS-A stability and solubility.
PS-A dissolved in THF degraded faster, while toluene was a more robust solvent, as displayed by a more steady rate of solvolysis.
Due to double exponential fitting, polystyrene would not be as stable in toluene as THF after 10 hours (Figure 6). This phenomenon requires more exploration as these organic solvents are not known to take part in the electron transfer needed to weaken polyolefin bonds. Possible impurities in the sample or shear stress might be the fundamental sources of PS-A degradation.
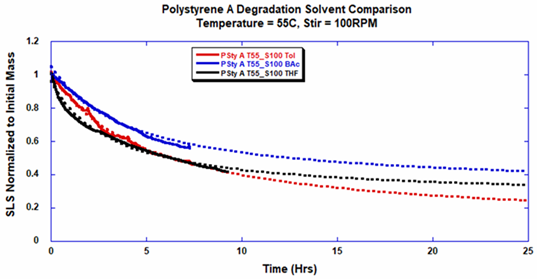
Figure 6. Polystyrene-A degradation in different solvents with stirring at 100 RPM was fitted with a double exponential. Image Credit: Yokogawa Fluence Analytics
Biopolymer degradation
The isoelectric point is the pH of the solvent or buffer system at which a biopolymer does not have a net charge. The isoelectric point of BSA is pH 4.8, and the protein is essentially insoluble owing to the absence of electrostatic repulsion at a pH of 4.8 (buffer system).
Following acidification or basification of the solvent or buffer system containing BSA, the protein either becomes positively (acidic buffer) or negatively (basic buffer) charged. This frequently leads to more colloidal stability and solubility through electrostatic repulsion.
The protein aggregates when thermal stress is at a pH of 5.85 and 5.21 (BSA is negatively charged). Acid-catalyzed hydrolysis happens at a pH ≤ 3.40, leading to protein fragmentation (generation of peptides), as noted by a smaller normalized molecular weight (Figure 7). There is a four-fold increase in protein degradation rate between pH 3.40 and pH 1.54, owing to a higher rate of acid-catalyzed hydrolysis.
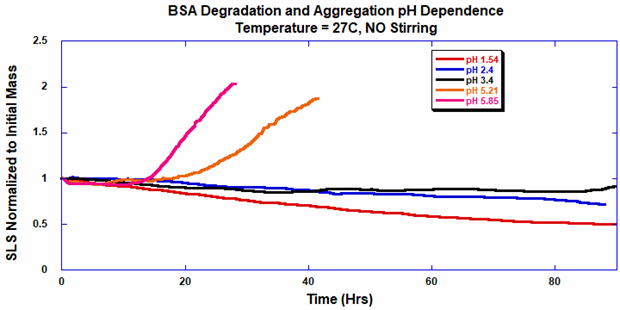
Figure 7. BSA degradation by acid hydrolysis at 27 °C with no stirring. Image Credit: Yokogawa Fluence Analytics
Conclusion
ARGEN™ was successfully used to calculate the molecular weight, clarify an undefined polymer's thermal, mechanical, and chemical stability, and track the solution behavior of bovine serum albumin under diverse pH conditions. The molecular weight of PS-A was made clear by Debye analysis utilizing data produced by the ARGEN™.
Information gathered on thermal, mechanical, and chemical stressors produced important data on polymer biopolymer stability.
ARGEN™ has proven itself as an essential instrument with the ability to examine the stability of organic polymers and biopolymers in real-time and give users the data required to accelerate the developmental process and formulation development.
About Yokogawa Fluence Analytics
Yokogawa Fluence Analytics, which was named as a Top 50 global advanced manufacturing startup by CB Insights, provides patented process analytics and control solutions to polymer and biopharmaceutical customers worldwide. Yokogawa Electric Corporation acquired Fluence Analytics in January 2023.
Yokogawa Fluence Analytics is a global leader in real-time polymer reaction monitoring and control, and its industry-leading ACOMP product is the only commercially available smart manufacturing system that continuously monitors and measures polymerization reactions.
The company’s biopharmaceutical product line includes a high-throughput static light scattering instrument called ARGEN. ARGEN can independently measure the stability of biopolymers under thermal, chemical, and mechanical (physical) stress, while also performing shelf-life stability studies at low temperatures.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.