Stability in the thermal, chemical, and mechanical domains are critical for proper development and delivery of therapeutic proteins. Proteins are frequently exposed to stress throughout purification, storage, and production operations through high-pressure liquid chromatography (HPLC), chemical modification, and lyophilization. Due to this, these processes can lead to aggregation, degradation or oxidation of the target protein making it ineffective.
Image Credit: Kateryna Kon/Shutterstock.com
ARGEN™ is the optimal high-throughput tool for understanding the impacts of thermal, chemical, and mechanical disturbances via changes in molecular weight. This permits fast vetting of constructs and buffer systems for the utmost stability. As a proof of concept (POC), ARGEN was used to track human insulin thermal stability at 50 °C, 53.5 °C, and 57 °C, generating data for time to dimer (TD) and time to tetramer (TT) determination.
ARGEN™: Smart & rapid therapeutic biopolymer development
ARGEN is a highly efficient tool that swiftly assesses the stability and viability of therapeutic proteins, peptides, and biopolymers. The tool utilizes a multi-stressor testing platform that uses static light scattering detection and intuitive data processing. These characteristics mean that researchers can generate biologic formulations up to 16 times faster.
How ARGEN™ works
ARGEN uses fixed angle (90°), SMSLS (simultaneous multiple sample light scattering) technology which produces fast, instantaneous, continuous data collection for determining both qualitative and quantitative characteristics of target molecules. The device has 16 independently controlled sample cells, allowing researchers to establish parameters of thermal, chemical, and mechanical (stirring) stress on every sample at the same time. This increases flexibility through the experimental design process.
ARGEN™ intuitive control software
The ARGEN control software boasts an easy-to-use interface for every aspect of experimental design and independent control of every cell for adjusting parallel parameters and processing data in real time.
Experimental methods
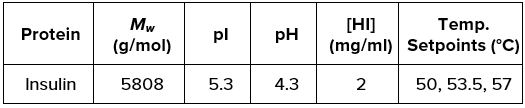
Researchers dissolved human Insulin (Sigma I2643) in sodium phosphate buffer at [HI] = 2 mg/mL, pH 4.3. All samples were assessed in isothermal conditions as described in Table 1 for a period not exceeding 48 hours.
Experimental parameters
Table 1. Experimental parameters for the thermal stability analysis of Human Insulin. Source: Yokogawa Fluence Analytics
Experimental results
Monitoring thermal aggregation of human insulin using ARGEN™
An understanding of the thermal stability of proteins is crucial for the proper development of protein-based drugs. Utilizing Human Insulin as a model, triplicate tests at every temperature set point (50 °C, 53.5 °C and 57° C) showed the potential for ARGEN™ to track HI aggregation uninterruptedly for up to 48 hours. As shown in Figure 1, at 50 °C, the protein persisted in a stable state for up to 24 hours, followed by aggregation at time stamps exceeding 24 hours. At 53.5 °C, Insulin formed a stable dimer for roughly 20 hours after an exponential increase in aggregation rate at time exceeding 20 hours. At 57 °C, insulin formed a stable higher order species with an exponential rise in aggregation rate 10 hours later.
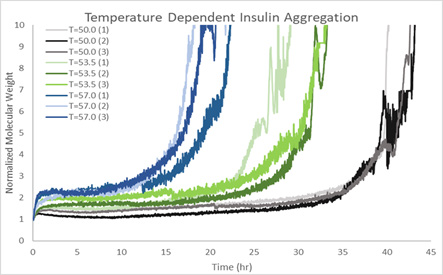
Figure 1. Mw/Mo (normalized molecular weight) vs. time (t) for human Insulin at 50 ⁰C, 53.5 ⁰C and 57 ⁰C (in triplicate). Image Credit: Yokogawa Fluence Analytics
Defining oligomeric state transitions of human insulin under thermal stress using ARGEN™
Defining the transition states of proteins to higher order oligomers and conditions affecting this behavior is essential for selecting the optimal construct and building solution conditions to optimize stability. The sensitivity of ARGEN™ to track the kinetics of monomer to dimer (Time to Dimer), dimer to tetramer (Time to Tetramer), and higher order transition states generates crucial insights into the stability and viability of therapeutic proteins when subjected to different thermal, chemical and mechanical stress conditions. Kinetic analysis of the information gathered on HI uncovered the time to dimerization (TD) (Figure 2), time to tetramerization (TT) (Figure 3), and aggregation rates (AR) at 50 ⁰C, 53.5 ⁰C and 57 ⁰C set points. As predicted, there was a reduction in TD and TT as temperatures rose, reflecting an increase in aggregation rates (AR). TT increased awareness of the instability of HI outside of the formation of a stable dimer species as suggested by the exponential aggregation rate (AR) (Figure 2).
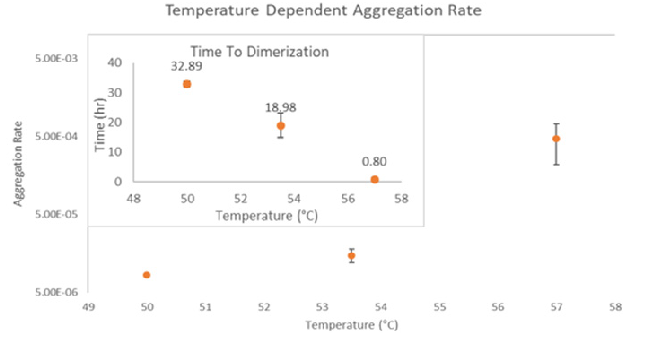
Figure 2. Time to Dimerization (inset) and aggregation rate (AR) of Human Insulin. Image Credit: Yokogawa Fluence Analytics
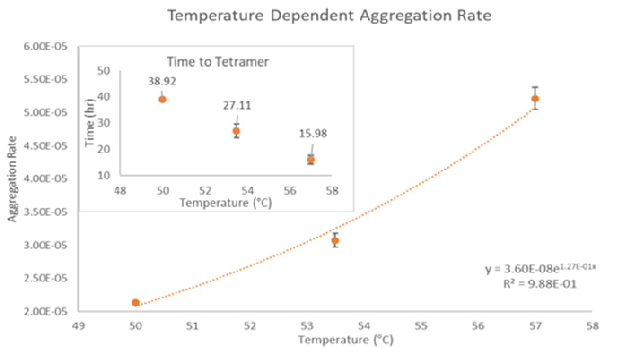
Figure 3. Time to Tetramerization (inset) and associated aggregation rate (AR) of Human Insulin. Image Credit: Yokogawa Fluence Analytics
Conclusion
These tests showcase the usefulness of ARGEN™ to characterize oligomeric states and transitions of Human Insulin when subjected to thermal stress. Time to dimerization (TD) and time to tetramerization (TT) assessments allowed the characterization of a stable dimer species, alongside insights into the underlying mechanisms and kinetics of higher order oligomer formation. While SEC facilitates the characterization of discrete oligomeric species’, integrated information gathered through ARGEN™ determines the formation of higher order species in real-time. Moreover, the results of these tests can be relayed to comprehend the impacts of buffer or solvent conditions, mechanical (stirring) stress and long-term storage (shelf-life) to verify the most powerful biotherapeutic construct(s).
About Yokogawa Fluence Analytics
Yokogawa Fluence Analytics, which was named as a Top 50 global advanced manufacturing startup by CB Insights, provides patented process analytics and control solutions to polymer and biopharmaceutical customers worldwide. Yokogawa Electric Corporation acquired Fluence Analytics in January 2023.
Yokogawa Fluence Analytics is a global leader in real-time polymer reaction monitoring and control, and its industry-leading ACOMP product is the only commercially available smart manufacturing system that continuously monitors and measures polymerization reactions.
The company’s biopharmaceutical product line includes a high-throughput static light scattering instrument called ARGEN. ARGEN can independently measure the stability of biopolymers under thermal, chemical, and mechanical (physical) stress, while also performing shelf-life stability studies at low temperatures.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.