Recombinant adenoviral vectors (rAAVs) have provided effective treatment outcomes for various monogenic disorders. They encode and deliver gene(s) to the host cell to alleviate or cure disease conditions.

Image Credit: Yokogawa Fluence Analytics
The canonical rAAV vector holds ≦ 4.7 kb single-stranded (ss) DNA within a 60-protein icosahedral capsid, and tropism is chosen from 13 known natural and supplementary unique hybrid serotypes enabling accurate tissue targeting.1,2
Successfully developing effective rAAV vectors can be difficult and necessitates a broad comprehension of the stability landscape under various thermal, chemical, storage, and mechanical (stirring) stress environments.
Quantitative and qualitative stability data facilitates the design of stabilizing solution condition(s) and the characterization of destabilizing effects from freeze-thaw cycling, manufacturing, processing, purification, processing, and storage.
The rAAV capsid stability tests in this article were undertaken to examine and observe full and empty rAAV capsid solution behavior under thermal stress utilizing the ARGEN tool.
Accelerating biotherapeutic formulation development
ARGEN is a benchtop laboratory tool that facilitates smart and rapid therapeutic biopolymer development. It's a static light scattering platform that addresses crucial demands in biopharmaceutical R&D. It enables researchers to:
- Replicate stress conditions, including temperature fluctuations and stirring, to observe formulation responses instantly.
- Observe biopolymer stability in situ and in real-time.
- Examine and contrast several formulations autonomously, decreasing the iterations required to achieve optimum stability.
- Predict aggregation and degradation propensities promptly, detecting issues before they develop.
- Evaluate shelf-life stability at low temperatures with long-term stability testing.
How ARGEN works
ARGEN uses fixed-angle (90°) simultaneous multiple sample light scattering technology to deliver fast, real-time, continuous data collection for categorizing qualitative and quantitative properties of in situ target molecules.
The tool comes with several independently controlled sample cells, enabling the operator to simultaneously determine each sample's thermal, chemical, and mechanical (stirring) stress parameters. This facilitates a highly adaptable method to experimental design.
ARGEN intuitive control software
The ARGEN control software features a user-friendly interface for experimental design and individual control of each cell for parallel parameter adjustment and real-time data management.
Materials and methods
Materials
rAAVs were made by triple transfection in HEK-293 cells and purified from crude cell lysate by ion exchange chromatography. Empty (without DNA) and full capsids (4.7 kb, ssDNA) were concentrated and dialyzed into 20 mM sodium phosphate and 180 mM NaCl at pH 7.3. Full and empty capsid samples were filtered utilizing a 0.22 µm cellulose acetate filter before the tests.
Methods
ARGEN is equipped with eight independent sample cells, enabling experimental parameter (temperature and stirring rate) alterations. Each sample cell was constructed with its own optical detector train.
The Zimm approximation determined the absolute molecular weight (Mw) of full and empty capsids. Mw(t) is the full or empty capsid’s molecular weight (average) defined by fluctuations in time-resolved light scattering intensity.
Mo is the full or empty capsid’s initial molecular weight before aggregation at t = 0. Mw(t)/Mo represents the temporal change in molecular weight (average) concerning the capsid’s native state.
All tests were undertaken in 1 cm pathlength, disposable cuvettes with 1 ml sample volumes.
Results
Monitoring rAAV capsid stability behavior under isothermal conditions
Categorizing viral rAAV capsid stability with or without DNA cargo and DNA ejection dynamics can enable a deeper understanding of DNA’s role in stabilizing capsid architecture. ARGEN was utilized for these tests to identify temporal changes in molecular weight (average) for the full and empty rAAV at 44 °C (Figure 1).
Empty capsids quickly produced higher-order aggregates caused by colloidal instability. This presents potential proof of DNA’s role in maintaining capsid architecture as proven by a fast increase in molecular weight (average). Analyzing full capsids established the complete ejection of DNA in ~5.6 hours (~20,000s) from the experiment’s onset.
Capsid protein aggregation proceeded slowly following the genome’s full evacuation. This phenomenon may happen because capsid proteins bind to the ejected DNA, which has a strong negative charge, preventing the formation of larger protein aggregates.
Additional experimentation is needed to fully comprehend the variations in aggregation rates of empty and full capsid-associated proteins.
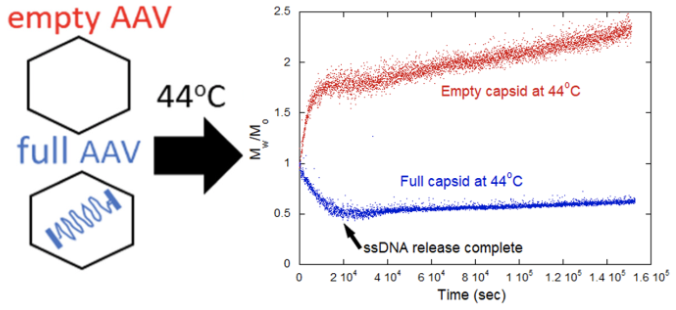
Figure 1. Normalized molecular weight Mw/M0 vs Time s for full and empty AAV capsids at 44 °C 3. Image Credit: Yokogawa Fluence Analytics
Molar mass determination of full and empty rAAV capsids using ARGEN
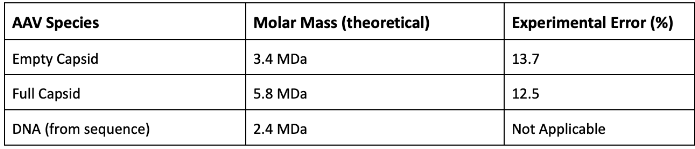
Successfully developing an rAAV vector system necessitates quantitative and qualitative classification of all vector components. Absolute molecular weights for the full and empty AAV capsids were defined utilizing the Zimm approximation, neglecting the interaction term (A2) from ARGEN’s generated data.
Table 1 highlights theoretical and experimental molar masses and related fractional errors. Fractional errors arise from concentration uncertainties (pooling of mixed and empty capsids), neglect of the interaction term (A2), and Mie scattering effects (angular dependence of non-Rayleigh scatterers).
Table 1. Theoretical and experimental molecular weights for rAAV components as determined by RALS ARGEN 3. Source: Yokogawa Fluence Analytics
The value of ARGEN in rAAV capsid stability research
ARGEN is ideal for in situ and real-time classification of solution stability of larger species such as rAAVs and LNP-RNA complexes.
This article highlights ARGEN’s capacity to interpret full and empty capsid stability variations and observe genome ejection. This information is crucial for optimizing rAAV formulations for stabilization throughout manufacture and storage.
Conclusion
Defining viral vector systems’ quantitative and qualitative properties ensures maximum efficacy and stability during processing, storage, and parenteral administration.
These experiments confirmed ARGEN’s capability to observe DNA ejection, solution behavior, and the rAAV capsid stability of full and empty vectors under thermal stress, while establishing the absolute molecular weights of AAV vector and vector components.
This information can provide a comprehensive stability environment for successfully developing AAV vector systems and LNP-RNA complexes.
References
- Issa, S.S. et al. (2023) 'Various AAV serotypes and their applications in gene therapy: An overview,' Cells, 12(5), p. 785. https://doi.org/10.3390/cells12050785.
- Haggerty, D.L. et al. (2019) 'Adeno-Associated Viral Vectors in Neuroscience research,' Molecular Therapy — Methods & Clinical Development, 17, pp. 69–82. https://doi.org/10.1016/j.omtm.2019.11.012.
- Jarand, C.W. et al. (2024) 'DNA released by Adeno-Associated virus strongly alters capsid aggregation kinetics in a physiological solution,' Biomacromolecules, 25(5), pp. 2890–2901. https://doi.org/10.1021/acs.biomac.4c00027.
About Yokogawa Fluence Analytics
Yokogawa Fluence Analytics, which was named as a Top 50 global advanced manufacturing startup by CB Insights, provides patented process analytics and control solutions to polymer and biopharmaceutical customers worldwide. Yokogawa Electric Corporation acquired Fluence Analytics in January 2023.
Yokogawa Fluence Analytics is a global leader in real-time polymer reaction monitoring and control, and its industry-leading ACOMP product is the only commercially available smart manufacturing system that continuously monitors and measures polymerization reactions.
The company’s biopharmaceutical product line includes a high-throughput static light scattering instrument called ARGEN. ARGEN can independently measure the stability of biopolymers under thermal, chemical, and mechanical (physical) stress, while also performing shelf-life stability studies at low temperatures.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.