Peptide-based pharmaceutical technology necessitates the delivery of therapeutic oligopeptides that are designed in a stable state for efficacious application. Following therapeutic peptide isolation or synthesis, peptides are frequently exposed to HPLC purification, synthetic modification, and preparatory treatments for storage over long periods. During any of these processes, spoilage can happen in the form of aggregation, degradation, or oxidation.
Image Credit: Danijela Maksimovic/Shutterstock.com
ARGEN (Aggregation Rate GENerator) allows users to correlate spoilage modes to changes in molecular weight. These correlations are produced continually and simultaneously with up to 15 other samples to quickly survey a process for conditions that result in maximal product quality. ARGEN was employed in this study to examine spoilage that resulted in increases in molecular weight stemming from aggregation or association. This article specifically addresses stability measures, including time to dimer (TD), and time to tetramer (TT). It is crucial to acknowledge that stable peptide products exhibit extremely long TD and TT values.
Methodology: Monitoring peptide aggregation with ARGEN
Researchers dissolved human insulin (Sigma I2643) in acidified deionized water to concentrations of 2 mg/mL. After they had dissolved, the pH was adjusted with 0.1 M HCl and 0.1 NaOH to pH 4.3. All samples were then placed into a disposable polystyrene cuvette and maintained at a constant temperature for up to 48 hours (Table).
Results: Monitoring insulin aggregation with ARGEN
Insulin exhibited noticeable aggregation over 48 hours, as shown in Figure 1. Similar to larger polypeptides studied before by ARGEN, insulin aggregates form a milky solution given enough time. Insulin remained relatively stable with a low aggregation signal for 24 hours at 50 °C, the study's lowest temperature. In contrast, samples maintained at higher temperatures demonstrated exponential aggregation.
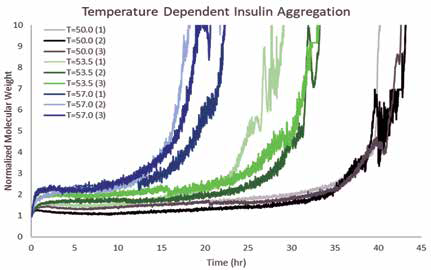
Figure 1. Insulin aggregates with Arrhenius-like kinetics for 48 hours. ARGEN observed a monotonic relationship between stability and temperature. Samples at 57 °C (blue) were
less stable than samples at 53.5 °C (green), which were less stable than samples held at 50 °C (black). Interestingly, many samples formed metastable dimer populations that existed for several hours before aggregating to higher-order multimers. Image Credit: Yokogawa Fluence Analytics
The aggregation kinetics of insulin incubated at 53.5 °C signaled a temperature-dependent destabilization. As the temperature increased to 57.0 °C, additional destabilization occurred. Kinetic data showed that metastable dimers formed in some samples following initial heating. In the most thermally-stressed samples, these dimers remained with little aggregation for multiple hours prior to aggregating to high-molecular-weight species. However, in less-stressed samples, the metastable species remained for a minimum of 24 hours before forming high-molecular-weight species.
Analysis of kinetic data demonstrates two intriguing measurements: time to dimer and time to tetramer. As previously stated, multiple samples swiftly reached a population of metastable dimers (Figure 1), with the high-temperature sample being the fastest to reach this population. The other samples did not form a population of metastable dimers, although they carried samples with scattering signals that were higher than the monomer population (Figures 1 & 2). Since the aggregation rate (AR) is typically calculated from the TD, the AR measurement for the high-temperature sample was inflated in comparison to other samples that did not exhibit the presence of metastable dimer population in their kinetic curves (Figure 2).
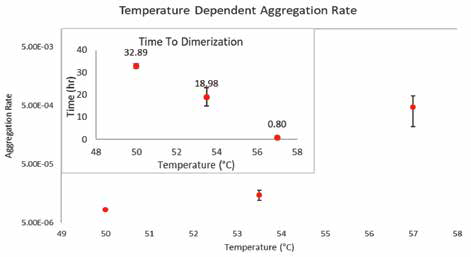
Figure 2. As temperature increases, time to dimerization decreases. The related measure, aggregation rate (AR), increases with respect to temperature. Image Credit: Yokogawa Fluence Analytics
To get a clearer understanding of every sample’s stability, AR is calculated using TT, as depicted in Figure 3. This measurement provides a more accurate assessment of all samples since it is independent of the formation of a metastable population. TT and the AR value obtained from it indicate the rate at which nuclei for higher-order aggregates are becoming widespread in solution. The importance of this assessment becomes clear only when examining the time-dependent, normalized molecular weight curves. For the three thermal conditions tested in this experiment, the ARs derived from TT demonstrated an exponential association where increasing temperature exponentially raises AR (Figure 3).
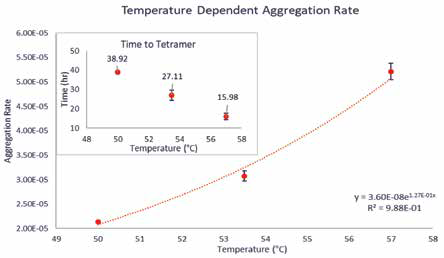
Figure 3. There is an approximately linear relationship between temperature and TT. This is reflected in the exponential relationship between AR and TT. This AR is different from the AR calculated from time to dimerization because it is blind to the rapid formation of metastable dimers; instead, it is based on the formation of unstable, higher-order aggregates that act as nuclei for further aggregation phenomena. Image Credit: Yokogawa Fluence Analytics
Conclusion
In this research, ARGEN was employed to continuously surveil a vital peptide-hormone-based pharmaceutical. ARGEN illustrates its usefulness in examining thermally-induced peptide aggregation as well as the importance of kinetic stability assessments when exploring protein and peptide aggregation phenomena. The findings of this experiment are straightforward, showing that higher temperatures lead to faster aggregation. However, detecting metastable populations would not have been possible without ARGEN’s continuous monitoring.
This study underscores the advantages of continuous monitoring for obtaining real-time data. Further studies using ARGEN could explore how formulation conditions, storage environments, or contaminant stressors affect peptide stability.
Table. Experimental Conditions for the Peptide of Interest, Insulin
| Peptide |
Molecular
Weight
(g/mol*) |
pI |
Experimental
pH |
Experimental
Concentration
(mg/mL) |
Experimental
Temperatures
(°C) |
| Insulin |
5,808 |
5.3 |
4.3 |
2 |
50, 53.5, 57 |
* The reported value for insulin’s molecular weight is that of the monomeric form. Insulin also exists in a hexameric form in solution
About Yokogawa Fluence Analytics
Yokogawa Fluence Analytics, which was named as a Top 50 global advanced manufacturing startup by CB Insights, provides patented process analytics and control solutions to polymer and biopharmaceutical customers worldwide. Yokogawa Electric Corporation acquired Fluence Analytics in January 2023.
Yokogawa Fluence Analytics is a global leader in real-time polymer reaction monitoring and control, and its industry-leading ACOMP product is the only commercially available smart manufacturing system that continuously monitors and measures polymerization reactions.
The company’s biopharmaceutical product line includes a high-throughput static light scattering instrument called ARGEN. ARGEN can independently measure the stability of biopolymers under thermal, chemical, and mechanical (physical) stress, while also performing shelf-life stability studies at low temperatures.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.