The development of biotherapeutics requires a thorough understanding of the effects of mechanical and physical stress. Instability and resulting aggregation can make them biologically inactive or cause an immunological response in patients. Stirring is among the most significant factors that disturb native biopolymer structure and lead to aggregation.
Image Credit: Nemes Laszlo/Shutterstock.com
Contact stirring, however, demonstrates just one type of stress that can occur while therapeutic proteins are purified, manufactured, and packaged. Overhead stirring, capillary shear stress, filtration, and peristaltic recirculation affect protein stability and cause aggregation.
As many or all of the defined mechanical and physical stressors can exist during the production of therapeutic proteins, it is essential to quantitatively evaluate the effects of each stressor.
ARGEN™: Smart and rapid therapeutic biopolymer development
ARGEN™ is a highly efficient instrument that can rapidly assess the stability and viability of therapeutic proteins, peptides, and biopolymers. The tool utilizes a multi-stressor testing platform enabled by static light scattering detection and intuitive data processing. These characteristics allow researchers to engineer biologic formulations up to 16 times faster.

Image Credit: Yokogawa Fluence Analytics
How ARGEN™ works
ARGEN™ uses fixed-angle (90°), simultaneous multiple-sample light scattering (SMSLS) technology, which enables quick, instantaneous, continuous data collection to characterize qualitative and quantitative attributes of target molecules. The device has 16 independently controlled sample cells, allowing users to simultaneously establish thermal, chemical, and mechanical (stirring) stress parameters on every sample. This enables an adaptable approach to experimental design.
ARGEN™ intuitive control software
The ARGEN™ control software has an intuitive interface for every aspect of experimental design and independent control of each cell, allowing simultaneous parameter adjustment and data processing in real-time.
Characterizing the impacts of different modes of stirring stress
The effects of various stirring modes demonstrate therapeutic biopolymers' stability and capacity to remain balanced in the purification and manufacturing processes. Every cell of ARGEN™ has a stepper motor fitted to a rotating magnet, which attaches to a micro stir bar at the bottom of the cuvette (contact stirring) or at the overhead (non-contact stirring) to mimic perturbations amid bioprocessing.
The stirring/agitation rate (0-2000 RPM) is controlled manually for accuracy using the ARGEN™ intuitive control software. Overhead stirring is carried out with a custom-engineered cuvette. The cuvette cap has a spindle from which a micro stir bar is suspended.
Sealed bearings in the cap permit the spindle and stir bar assembly to rotate freely and synchronously with the magnetic stepper motor.
Figure 1 shows the discrepancy in aggregation rates (slope of Mw/Mo) between contact stirring and non-contact stirring. Changes in Mw/Mo and aggregation were noted at the beginning of all (triplicate) contact stirring experiments. While the protein was stable for ~10 hrs when subjected to non-contact stirring, this data explicitly demonstrates that altering the mode of stirring stress applied to the monoclonal antibody leads to rather uneven aggregation rates and profiles that can be crucial during the vetting process and while determining the most viable construct.
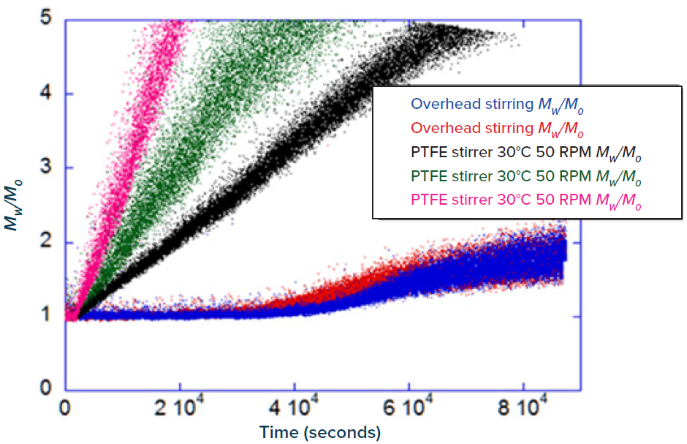
Figure 1. Change in normalized molecular weight (Mw/Mo) vs. time (seconds) of a monoclonal antibody when subjected to non-contact (overhead) vs. contact stirring. Image Credit: Yokogawa Fluence Analytics
Conclusion
Comprehending the effects of stirring and shear stress on biopolymers throughout manufacturing and processing is critical for speeding up development. The capacity of ARGEN™ to mimic stirring stress while concurrently monitoring oligomerization or degradation states is unique and enables treatments to reach customers.
This case study demonstrates the impacts of various modes of stirring stress experienced during bioprocessing on a monoclonal antibody.
Real-time monitoring of changes in normalized molecular weight showed that contact stirring would not be a preferred approach, as the protein aggregated instantly. This data is vital for vetting constructs to find the ideal candidate and designing processing equipment to reduce the impacts and perturbations experienced in bioprocessing.
About Yokogawa Fluence Analytics
Yokogawa Fluence Analytics, which was named as a Top 50 global advanced manufacturing startup by CB Insights, provides patented process analytics and control solutions to polymer and biopharmaceutical customers worldwide. Yokogawa Electric Corporation acquired Fluence Analytics in January 2023.
Yokogawa Fluence Analytics is a global leader in real-time polymer reaction monitoring and control, and its industry-leading ACOMP product is the only commercially available smart manufacturing system that continuously monitors and measures polymerization reactions.
The company’s biopharmaceutical product line includes a high-throughput static light scattering instrument called ARGEN. ARGEN can independently measure the stability of biopolymers under thermal, chemical, and mechanical (physical) stress, while also performing shelf-life stability studies at low temperatures.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.