Intravital brain imaging is a powerful method for observing neurons, glial cells, and their surrounding environments in living animals. It facilitates the study of neurological diseases, drug efficacy, and the immune system. Two-photon microscopy stands out as an indispensable tool for in vivo imaging among the various brain imaging methods.
Two-photon microscopy enables deep-tissue imaging by utilizing two lower-energy photons to excite fluorophores due to the reduced scattering of longer-wavelength infrared light. This leads to clearer images with minimal background noise from out-of-focus light.
The integration of tunable two-photon lasers further refines imaging by adjusting excitation wavelengths, allowing for the effective excitation of a broader range of fluorophores. This enhancement offers greater versatility and comprehensive imaging across various biological contexts.
Conventionally, the cranial imaging window (CIW) has been employed for brain imaging. This approach involves creating a hole in the skull and placing a transparent window over the exposed brain tissue, allowing observation through microscopy.
In vivo brain imaging has been primarily limited to cortical regions, with considerable challenges in visualizing deeper brain structures, such as the hippocampus. These limitations hinder a comprehensive exploration of the complex dynamics of deeper brain regions in living organisms.
The Cranial Imaging Cannula (CIC) is an innovative surgical strategy that facilitates real-time imaging of deep brain regions, including the hippocampus and hypothalamus—key areas for researching neurodegenerative diseases and neural dynamics.
The CIC enables prolonged observation of deep brain structures with minimal tissue damage through precise cannula insertion into the brain, making it ideal for longitudinal studies. This strategy can track drug effects or disease progression over extended periods, such as weeks or months.
This study employed tunable two-photon microscopy alongside the CIW technique to observe neuronal activity in Thy1-GCaMP6f transgenic mice. GCaMP6f is the fastest calcium indicator for detecting cytoplasmic free calcium.
In these mice, GCaMP6f is expressed under the Thy1 promoter, enabling detection of neuronal activity via calcium fluctuations corresponding to neuronal firing. This setup facilitated the visualization of GCaMP6f fluorescence in cortical neurons.
A fluorophore-conjugated CD31 antibody (CD31-Setau647) was also used for in vivo blood vessel labeling. The tunable two-photon laser’s excitation wavelength was optimized to simultaneously capture both GCaMP6f and Setau647 signals.
The depth of brain visualization achievable was then explored using the CIW technique combined with two-photon microscopy, comparing it to the CIC method for accessing deeper brain regions. This comparison allowed for the assessment of each technique’s effectiveness, particularly for visualizing deep brain areas.
Materials & methods
Mouse models
Thy1-GCaMP6f transgenic mice were used to monitor neuronal activity. These mice are genetically modified to express GCaMP6f under the Thy1 promoter, allowing for stable and reproducible fluorescence in neurons. Thy1-M transgenic mice were used for imaging cortical neurons, and C57BL/6N mice were selected for visualizing the hippocampal CA1 region.
Cranial imaging window
The CIW technique was used for long-term, stable brain cortex imaging. Mice were anesthetized with a mixture of Zoletil, Rumpun, and saline, administered via intramuscular injection to ensure adequate sedation and pain management.
Once anesthetized, the mice were placed in a stereotaxic frame to precisely target the cortex. A craniotomy was performed, followed by placing a cover glass over the exposed brain and securing it with optical bond and dental cement.
Cranial imaging cannula surgery
Following anesthesia, mice were placed in a stereotaxic frame, and a 3 mm diameter hole was drilled into the skull using the same procedure as CIW surgery to allow precise targeting of the hippocampal CA1 region.
After exposing the brain, the meninges and part of the cortex were removed through a cranial opening using suction to reveal the hippocampus. Once the bleeding had stopped, a cranial imaging cannula was inserted and covered with a 3 mm cover glass. Except for the cannula, the incision area was secured with fast-curing adhesive and dental acrylic resin.
Blood vessel labeling
Fluorophore-conjugated antibodies (anti-CD31-Setau647, IVIM Technology) were administered via intravenous injection to visualize blood vessels in live mice. Two hours post-injection, intravital confocal, and two-photon microscopy (IVM-CM3) were used to capture images of the blood vessels.
Two-photon imaging
After the surgical procedure, mice were placed in a cage to recover and monitored for at least 2~4 weeks. During this period, inflammation was controlled via injection of Rimadyl (Carprofen, Zoetis).
To visualize blood vessels, the IVI Tag TM In Vivo Labeling Kit (IVIM Technology, Korea Republic) was used. Under anesthesia, 25 μL of its endothelial cell labeling solution was injected intravenously via the tail vein.
One hour after injection, the mouse was positioned on a stereotaxic stage with a heating function in the IVM-CM3 intravital microscope (IVIM Technology, Korea Republic).
For stability during imaging, mice were placed in a stereotaxic frame. Two-photon imaging was conducted using intravital confocal and two-photon microscopy (IVM-CM3).
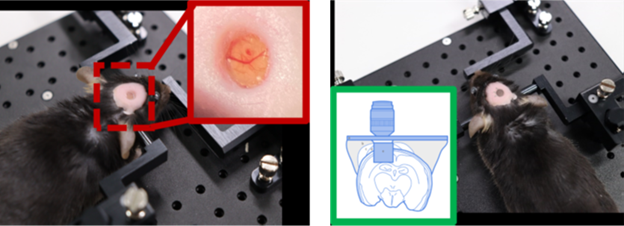
Figure 1. Mouse animal models implanted with (A) Cranial imaging window, and (B) Cranial imaging cannula. Image Credit: Scintica Instrumentation Inc.
Results
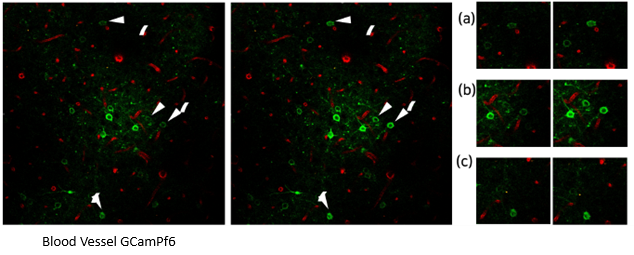
Figure 2. Visualization of GCaMP Expression. Image Credit: Scintica Instrumentation Inc.
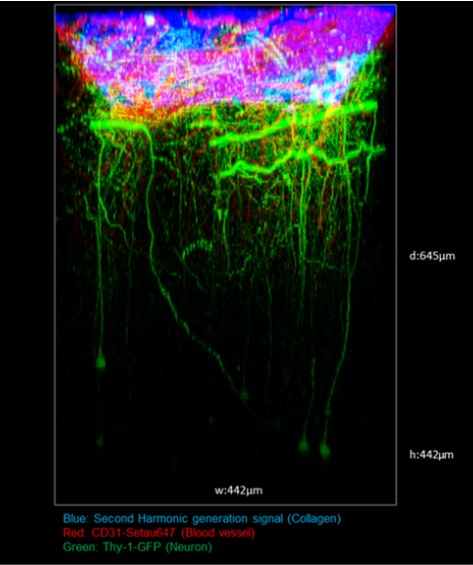
Figure 3. Two-Photon Brain Imaging in CIW-Implanted Thy1-M-GFP Mice. Image Credit: Scintica Instrumentation Inc.
Thy1-GCaMP6f mice express an endogenous GFP signal to detect Ca2+ ion-dependent neuronal activity. When neurons are activated, GCaMP6f binds to calcium ions and quickly expresses its endogenous GFP. This makes it advantageous for studying neuronal cell activity. To visualize real-time changes in neuronal activity, this mouse species was used after CIW implantation.
Data (Figure 1A and Figure 2A) indicates that some neurons had increased GFP, suggesting that the CIW-implanted mouse model can acquire real-time cellular dynamics in the brain.
In Thy1-M-GFP mice implanted with a CIW, brain depth was measured with a 920 nm two-photon laser. Second harmonic generation signals from collagen indicated its presence in the superficial layers. Blood vessels were noted below the collagen layer, with larger vessels in the upper regions and microvessels becoming more predominant at increased depths.
Neurons labeled with Thy1-M-GFP were successfully visualized, showing that two-photon microscopy in combination with CIW technology allowed observation of up to around 600 μm within the cortical area.
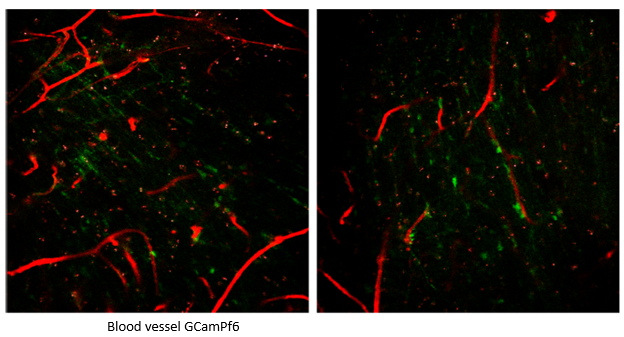
Figure 4. Two-Photon Hippocampal CA1 Imaging in CIC-Implanted Mice. Image Credit: Scintica Instrumentation Inc.
A CIC was implanted in mice to overcome imaging depth limitations and visualize deep brain regions. Utilizing a 920 nm two-photon laser, blood vessels in the hippocampal CA1 region of the CIC-implanted mice were successfully observed.
These findings demonstrate that the CIC technique enables real-time imaging of living mice's deep brain regions, such as the hippocampus. This advancement enhances the observation of cortical neuronal activity, blood vessels, and deeper brain areas, making it an invaluable tool for studying neurological diseases and drug responses in previously inaccessible brain areas.
References and further reading:
- Dana, H., et al. (2014). Thy1-GCaMP6 Transgenic Mice for Neuronal Population Imaging In Vivo. PLoS ONE, 9(9), p.e108697. https://doi.org/10.1371/journal.pone.0108697.
- Bradley, J.E., Ramirez, G. and Hagood, J.S. (2009). Roles and regulation of Thy-1, a context-dependent modulator of cell phenotype. BioFactors, [online] 35(3), pp.258–265. https://doi.org/10.1002/biof.41.
- Benninger, R.K.P. and Piston, D.W. (2013). Two-Photon Excitation Microscopy for the Study of Living Cells and Tissues. Current Protocols in Cell Biology, 59(1), pp.4.11.1–4.11.24. https://doi.org/10.1002/0471143030.cb0411s59.
 About Scintica Instrumentation Inc.
About Scintica Instrumentation Inc.
Scintica Instrumentation Inc., a high value distributor of scientific medical equipment, was created as a joint venture between two companies, Indus Instruments and ONS Projects Inc., both with long standing experience in the medical device instrumentation field. Indus Instruments is an engineering and manufacturing company with excellence in designing and producing sophisticated products for both medical and other high-tech clients in aerospace, chemical and oil and gas industries. ONS Projects Inc. is a life science investment and marketing company built on the foundation of two other successful manufacturing companies in the laboratory instrumentation field,
The principals of the two companies each have more than 25 years of experience of manufacturing, selling and supporting scientists in their research around the world. Our team consists of scientists, applications experts, engineers and sales professionals from a cross section of backgrounds, who excel at simplifying transactions and ensuring that scientists have the best equipment for achieving research excellence.
At Scintica Instrumentation, we distribute for selected manufacturers from all over the world and represent them in multiple countries including the United States, Canada, and Europe, as well as in Asia through a network of authorized sub-distributors.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.