Stroke is the second leading cause of death globally and a major contributor to adult disability. According to the World Health Organization, approximately 15 million people suffer a stroke annually—5 million of whom die, and another 5 million are left with permanent disabilities.

M-Series MRI. Image Credit: Scintica Instrumentation Inc.
The incidence of stroke is expected to rise in the coming years, driven by aging populations and rising rates of risk factors such as hypertension and obesity. Despite advancements in medical management and neurosurgical techniques, no effective neuroprotective agents currently exist to improve outcomes after ischemic stroke.
Nanoparticles, particularly liposomes, have been proposed as a possible neuroprotective strategy for treating ischemia-reperfusion injury. These biocompatible and biodegradable liposomes can deliver therapeutics across the blood-brain barrier, increasing drug accumulation at diseased sites.
Research has shown statins, specifically atorvastatin, to be effective in treating cardiovascular diseases and have potential as a neuroprotective agent. A recent study titled “Treatment of Ischemic Stroke by Atorvastatin-loaded PEGylated Liposome,” conducted by researchers at Chonnam National University Medical School and Hwasun Hospital showed that PEGylated liposomes loaded with atorvastatin (LipoStatin) efficiently accumulated at the site of cerebral ischemic injury.
Images captured with the Aspect Imaging M7 preclinical MRI and the Sedecal SuperArgus preclinical PET/CT system displayed a reduction in infarct volume (anatomical MRI), improved neurological function recovery, and enhanced brain metabolism. This was evidenced by a significant increase in the uptake of the clinically used radiotracer 18F-fluoro-deoxyglucose.
At the study endpoint, ex vivo analysis of the rat brain revealed that treatment with lipoStatin resulted in notable anti-inflammatory effects, recovery of blood-brain barrier breakdown, and endothelial dysfunction. This was evidenced by reduced extravasation of Evans blue, measured via ex vivo fluorescence imaging using systems such as the Vilber Newton bioluminescence and fluorescence system.
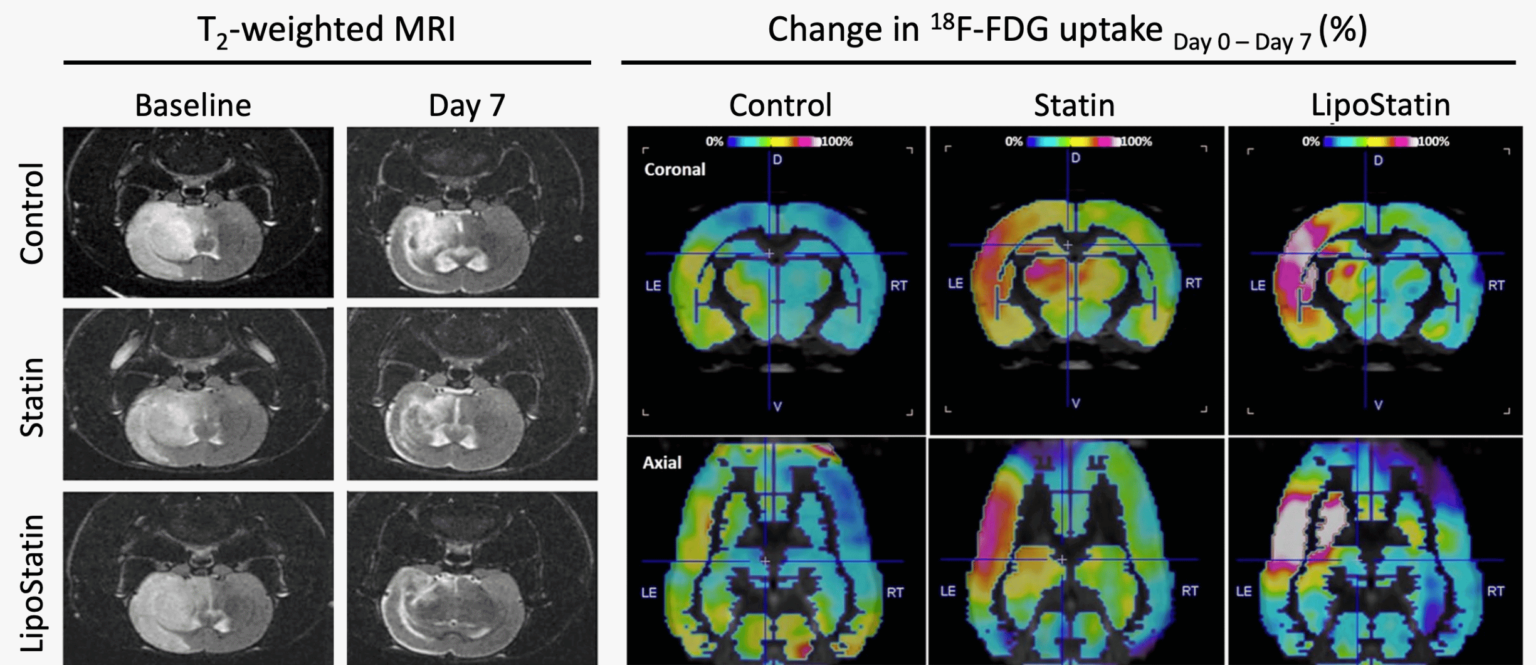
Image Credit: Adapted from Thomas, R.G., Transl Stroke Res (2023). https://doi.org/10.1007/s12975-023-01125-9
References and further reading:
Thomas, R.G., et al. (2023). Treatment of Ischemic Stroke by Atorvastatin-Loaded PEGylated Liposome. Translational Stroke Research. https://doi.org/10.1007/s12975-023-01125-9.
 About Scintica Instrumentation Inc.
About Scintica Instrumentation Inc.
Scintica Instrumentation Inc., a high value distributor of scientific medical equipment, was created as a joint venture between two companies, Indus Instruments and ONS Projects Inc., both with long standing experience in the medical device instrumentation field. Indus Instruments is an engineering and manufacturing company with excellence in designing and producing sophisticated products for both medical and other high-tech clients in aerospace, chemical and oil and gas industries. ONS Projects Inc. is a life science investment and marketing company built on the foundation of two other successful manufacturing companies in the laboratory instrumentation field,
The principals of the two companies each have more than 25 years of experience of manufacturing, selling and supporting scientists in their research around the world. Our team consists of scientists, applications experts, engineers and sales professionals from a cross section of backgrounds, who excel at simplifying transactions and ensuring that scientists have the best equipment for achieving research excellence.
At Scintica Instrumentation, we distribute for selected manufacturers from all over the world and represent them in multiple countries including the United States, Canada, and Europe, as well as in Asia through a network of authorized sub-distributors.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.