Patients with Glioblastoma (GBM), a highly aggressive malignant brain tumor, have an extremely poor prognosis due to a lack of viable treatment choices. Since being named the “breakthrough of the year” by Science in 2013, immunotherapy has transformed the treatment of solid cancers.

M-Series MRI. Image Credit: Scintica Instrumentation Inc.
This includes small-molecule immune checkpoint medications that target either PD-1 (nivolumab, pembrolizumab) or its ligand (PD-L1, durvalumab, atezolizumab).
Immune checkpoint inhibitors have lately demonstrated encouraging outcomes across various tumor types. PD-L1 expression, as seen through histopathological analysis, serves as a valuable biomarker for stratifying patients who will benefit the most from the treatment.
Unfortunately, only moderate and unexpected responses for GBM patients have been documented thus far, a disappointing outcome that might be related partly to tumor heterogeneity and evolution, i.e., geographic and temporal alterations in immunological checkpoints, and our inability to accurately measure these changes.
There is growing evidence that immune-positron emission tomography (immuno-PET) can provide a real-time quantitative readout of PD-L1 expression levels and longitudinal measurements that monitor dynamic changes in PD-L1 expression without needing repeated biopsies.
In this study, the research team led by Dr. Gabriela Kramer-Marek at The Institute of Cancer Research in London explained the suitability of the affibody molecule (ZPD-L1) radiolabeled with fluorine F-18 and gallium Ga-68 to noninvasively evaluate PD-L1 expression levels in orthotopic xenograft GBM models (Figure 1).
Both 18F-AlF-NOTA-ZPD-L1 and 68Ga-NOTA-ZPD-L1 showed preferential accumulation in PD-L1-positive tumors and detected GBM brain tumors with good contrast, supporting T2-weighted MRI tumor delineation.
The use of Scintica Instrumentation’s M-Series compact MRI range was critical for quantifying tumor volume, monitoring growth and infiltrative phenotype following intracranial injection of human glioblastoma U87-MGvIII cells or Glioma GCGR-E55 stem cells in mice, and providing a decision-making tool to plan the timing of the immuno-PET experiment.
The Aspect Imaging M-series range provides cost-effective, low-maintenance, and compact MRI solutions for mice (M3), rats (M7), and non-human primates (M12) based on a unique permanent magnet design and an efficient workflow supported by integrated physiological monitoring and an easy-to-use operating system.
The M-Series’ field strength (1 Tesla) is sufficient for quick screening of tumor formation, progression, and response assessment, making it ideal for lung imaging and developing clinically applicable functional and/or molecular imaging techniques.
This result suggests that anti-PD-L1 antibody-based immuno-PET could be a useful biomarker for the non-invasive classification of GBM patients and for optimizing PD-1/PD-L1 checkpoint blockade therapy.
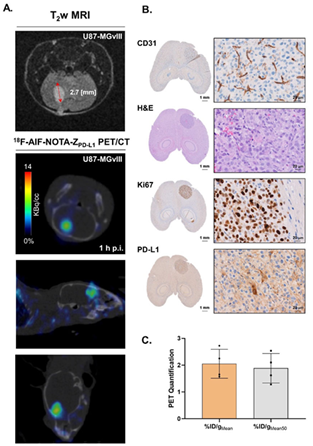
Figure 4. (A) T2-weighted MRI scans of intravenous injections of 18F-AlF-NOTA-ZPD-L1 1 h postinjection in U87-MGvIII orthotopic tumours. The mice received 0.2-0.76 MBq of radioconjugate. (B) shows histopathological validation of the tumour vascularisation (CD31), location (HE), proliferation status (Ki67) and PD-L1 expression. (C) PET quantification of 18F-AlF-NOTA-ZPD-L1 tumour uptake. Image Credit: Scintica Instrumentation Inc.
References
Sharma, G., et al. (2023). Immuno-PET Imaging of Tumour PD-L1 Expression in Glioblastoma. Cancers, [online] 15(12), p.3131. https://doi.org/10.3390/cancers15123131.
About Scintica Instrumentation Inc.
Scintica Instrumentation Inc., a high value distributor of scientific medical equipment, was created as a joint venture between two companies, Indus Instruments and ONS Projects Inc., both with long standing experience in the medical device instrumentation field. Indus Instruments is an engineering and manufacturing company with excellence in designing and producing sophisticated products for both medical and other high-tech clients in aerospace, chemical and oil and gas industries. ONS Projects Inc. is a life science investment and marketing company built on the foundation of two other successful manufacturing companies in the laboratory instrumentation field,
The principals of the two companies each have more than 25 years of experience of manufacturing, selling and supporting scientists in their research around the world. Our team consists of scientists, applications experts, engineers and sales professionals from a cross section of backgrounds, who excel at simplifying transactions and ensuring that scientists have the best equipment for achieving research excellence.
At Scintica Instrumentation, we distribute for selected manufacturers from all over the world and represent them in multiple countries including the United States, Canada, and Europe, as well as in Asia through a network of authorized sub-distributors.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.