The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 and has since rapidly spread around the world, causing the coronavirus disease 2019 (COVID-19) pandemic in its wake. The most affected population during the first wave of the pandemic were the elderly and frail who, as a result, were prioritized to receive COVID-19 vaccines when they became available to the public at the beginning of 2021.
 Study: Robust Immune Response to The BNT162b mRNA Vaccine in An Elderly Population Vaccinated 15 Months After Recovery From COVID-19. Image Credit: Olena Yakobchuk / Shutterstock.com
Study: Robust Immune Response to The BNT162b mRNA Vaccine in An Elderly Population Vaccinated 15 Months After Recovery From COVID-19. Image Credit: Olena Yakobchuk / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The Pfizer-BioNTech vaccine was one of the first COVID-19 vaccines to receive emergency authorization (EUA) in the United States, as well as in several other nations around the world. Developed on a messenger ribonucleic acid (mRNA) platform, this vaccine showed high vaccine efficacy (VE) in Phase III clinical trials and has since been found to retain its efficacy in real-world circumstances.
The waning of vaccine-induced immunity has been a concern. Conversely, prior infection with SARS-CoV-2 before getting the vaccine has been reported to boost antibody responses very effectively. In the elderly person, the weakening of the immune system with age is linked to a poor immune response to the vaccine after one dose.
Two doses, however, elicit a high level of immunoglobulin G (IgG) antibodies and neutralizing antibodies in uninfected individuals. The focus of the current study was the effect of one dose on an elderly individual with a history of natural infection with SARS-CoV-2 since it is biologically plausible that the vaccine dose will boost the antibody titer.
There is also little knowledge of how immune molecules react to vaccination after prior infection, as well as in SARS-CoV-2-naïve individuals.
About the study
The researchers used an outbreak in a convent where 16 elderly nuns, with a mean age of 81, developed and recovered from mild COVID-19. All study participants had other comorbidities and received one dose of the Pfizer vaccine 15 months after recovery.
The immune responses in this group were compared to those of another younger healthy cohort without a history of prior infection. In this other group, the mean age of the participants was 59 years, and all of these individuals had received two doses of the same vaccine at a five-week interval.
The researchers measured ten cytokines, finding that gamma-interferon (IFNγ) and CXCL10 rose in both groups within a day of vaccination. Whereas IFNγ remained high for a week in both groups, CXCL10 levels remained high only in the recovered group, thus indicating that the immune response was continuing over the long term.
In the naïve group, interleukin 16 (IL-16) levels fell after vaccination, while IL-8 levels were higher from the seventh post-vaccine day.
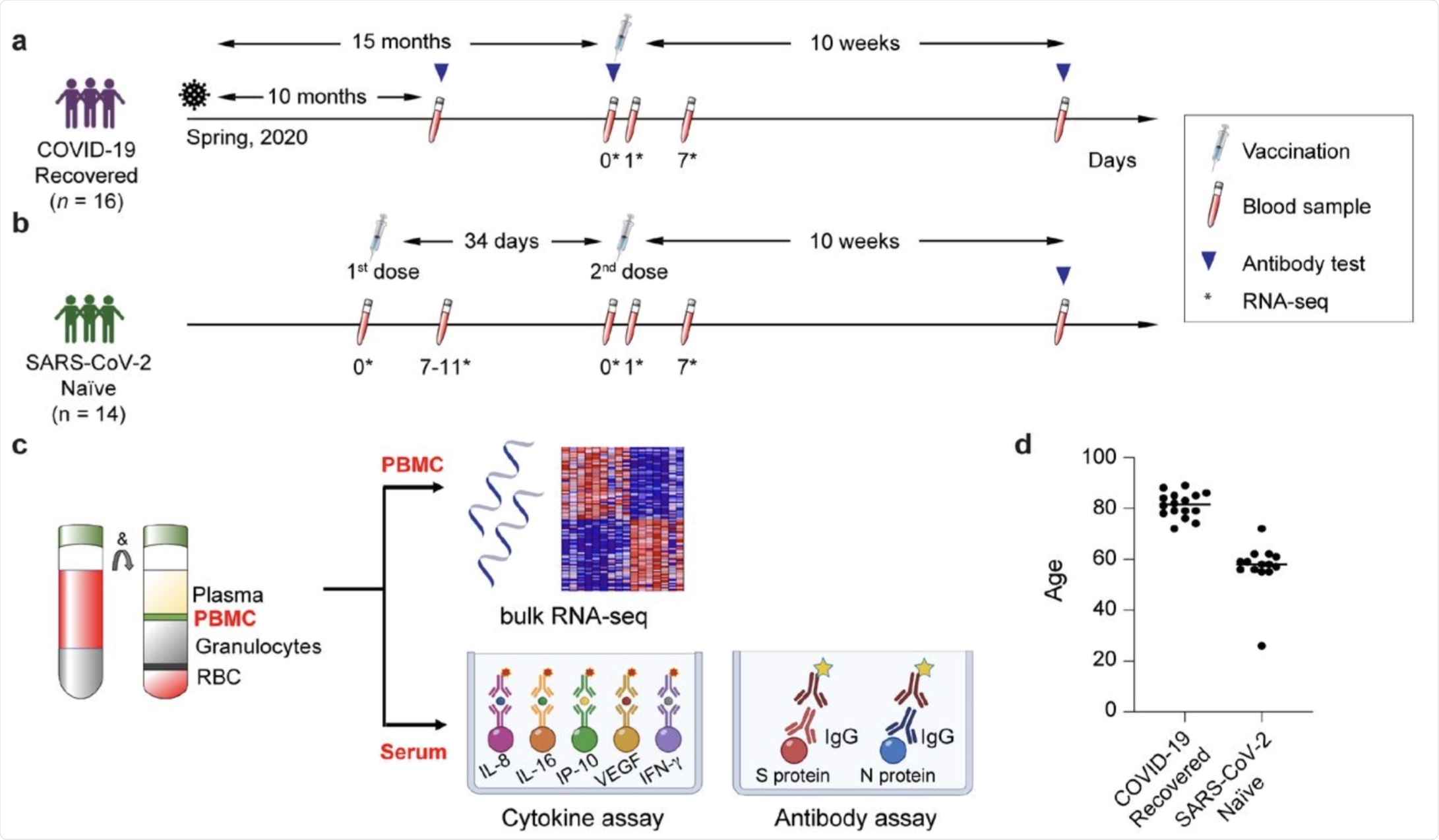 a-c. Schematic presentation of the experimental workflow. (a) elderly population recovered from COVID-19; (b) SARS-CoV-2 antigen-naïve population; the syringes indicate vaccinations; the test tubes indicate the blood drawing and the triangles the antibody testing. The asterisks indicate the days at which samples for RNA-seq was collected. (c) Peripheral blood mononuclear cells (PBMCs) were purified from blood drawn prior to and after the vaccination and RNA-seq was performed at a depth of 240 million reads per sample. d. Age distribution of participants in each cohort.
a-c. Schematic presentation of the experimental workflow. (a) elderly population recovered from COVID-19; (b) SARS-CoV-2 antigen-naïve population; the syringes indicate vaccinations; the test tubes indicate the blood drawing and the triangles the antibody testing. The asterisks indicate the days at which samples for RNA-seq was collected. (c) Peripheral blood mononuclear cells (PBMCs) were purified from blood drawn prior to and after the vaccination and RNA-seq was performed at a depth of 240 million reads per sample. d. Age distribution of participants in each cohort.
Antibody response after vaccination
The elderly cohort showed anti-spike IgG with a mean level of about 700 U/mL at 10 and 15 months from the time of infection when they received one dose of the vaccine.
Ten weeks from the single vaccine dose, anti-spike antibody titers ranged from 17,000 to 121,000 U/mL in the previously exposed cohort. This was comparable to a rise from 500 to 3,700 U/mL after two vaccine doses in the naïve cohort. Antibody titers in the elderly with prior infection were thus 20 times higher than that which was detected in the younger naïve cohort.
Transcriptomics response
The immune molecules found in the Pfizer vaccine response in the recovered cohort as compared to the naïve cohort were most different at the seventh day post-vaccination.
In the former group, over 160 genes were upregulated two-fold within a day from the single dose of vaccine, with 108 remaining activated at day 7. By one week following transcription, almost 900 genes had been induced, with the first activated genes related to the interferon and JAK-STAT pathways.
In the naive group, about 170 genes were activated within a day of the second dose, and only 5 remained elevated at day 7. The genes induced by vaccination were mostly related to chemokine and cytokine signaling pathways.
The differences seen here indicate that the vaccine induced a long period of transcriptional changes in individuals who had a prior history of COVID-19. In fact, these individuals exhibited almost 550 genes that were induced two-fold or more in the first week following their single dose of the vaccine.
Interestingly, the immediate response in both groups was similar to that occurring after vaccination with the inactivated flu virus.
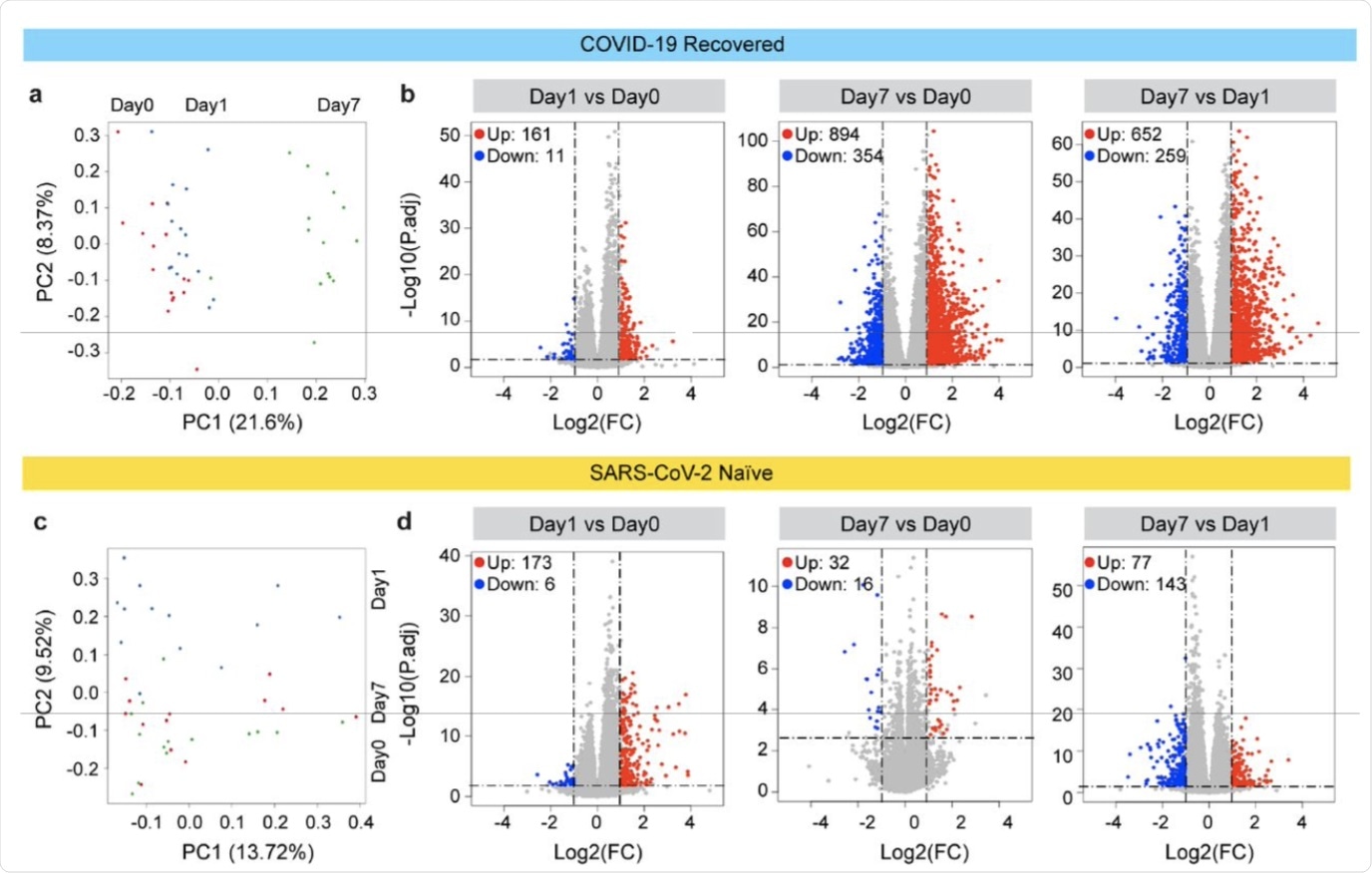 Principal-component analysis (PCA) of transcriptomes generated prior to (day 0) and after the vaccination (days 1 and 7) from COVID-19 recovered individuals. The variation in the global gene expression profiles across the three time points is shown. Principal components 1 (PC1) and 2 (PC2), which represent the greatest variation in gene expression, are shown. b. Volcano plots of DEGs comparing Day1 versus Day0, Day7 vs Day0 and Day7 vs Day1 in the COVID-19 recovered cohort. DEGs (adjusted p-value, P.adj < 0.05) with a log2 fold change (FC) of more than 1 or less than -1 are indicated in red and blue, respectively. Non-significant DEGs are indicated in gray. The numbers of upregulated and downregulated genes are listed in Supplementary Tables 4-6. c. PCA of transcriptomes from the SARS-CoV-2 antigen-naïve cohort prior to (day0) and day1 and day7 after the second (booster) vaccination. d. Volcano plot of DEGs comparing Day1 versus Day0, Day7 vs Day0 and Day7 vs Day1 in SARS-CoV-2 naïve cohort. The numbers of upregulated and downregulated genes are listed in Supplementary Tables 7-9. Volcano plots depict differentially expressed cytokines and chemokine at day 0, 1 and 7 of 1st vaccination of COVID-19 recovered cohort (a) and 2nd vaccination of SARS-CoV-2 naïve cohort (b). Red dots indicate significant upregulation; blue dots indicate significant downregulation (p-value < 0.05). See also Supplementary Figure 1 and Supplementary Tables 2.
Principal-component analysis (PCA) of transcriptomes generated prior to (day 0) and after the vaccination (days 1 and 7) from COVID-19 recovered individuals. The variation in the global gene expression profiles across the three time points is shown. Principal components 1 (PC1) and 2 (PC2), which represent the greatest variation in gene expression, are shown. b. Volcano plots of DEGs comparing Day1 versus Day0, Day7 vs Day0 and Day7 vs Day1 in the COVID-19 recovered cohort. DEGs (adjusted p-value, P.adj < 0.05) with a log2 fold change (FC) of more than 1 or less than -1 are indicated in red and blue, respectively. Non-significant DEGs are indicated in gray. The numbers of upregulated and downregulated genes are listed in Supplementary Tables 4-6. c. PCA of transcriptomes from the SARS-CoV-2 antigen-naïve cohort prior to (day0) and day1 and day7 after the second (booster) vaccination. d. Volcano plot of DEGs comparing Day1 versus Day0, Day7 vs Day0 and Day7 vs Day1 in SARS-CoV-2 naïve cohort. The numbers of upregulated and downregulated genes are listed in Supplementary Tables 7-9. Volcano plots depict differentially expressed cytokines and chemokine at day 0, 1 and 7 of 1st vaccination of COVID-19 recovered cohort (a) and 2nd vaccination of SARS-CoV-2 naïve cohort (b). Red dots indicate significant upregulation; blue dots indicate significant downregulation (p-value < 0.05). See also Supplementary Figure 1 and Supplementary Tables 2.
Implications
The current study indicates that with one dose of the Pfizer vaccine, elderly people who had recovered from mild COVID-19 15 months before responded with a robust antibody response. This belies the common perception of immunosenescence with aging, coupled with chronic systemic inflammation at a low level (inflammaging), both of which are linked to suboptimal vaccine response.
The immune response in this group was even superior to that of a younger group of naïve individuals, despite the fact that this latter group received both doses of the vaccine.
The question remains as to which time interval is best to boost the antibody response in a cohort of people previously exposed to SARS-CoV-2. Earlier studies have shown that the immune response in people younger than 50 years who receive one dose of the vaccine within six months of infection is comparable to that of the full-fledged two-dose regimen in an age-matched cohort.
Other researchers demonstrated the superior protection provided by an infection-plus-single booster dose against the Delta variant compared to two doses of the vaccine. There is still no consensus on whether one or two vaccine doses are necessary for this pre-exposed cohort.
Currently, a third booster dose of the vaccine is being released and is being shown to increase antibody levels markedly. These findings are particularly true when comparing the two-dose regimen in adults 60 years or older, with the second dose having been taken five or more months before. This booster is very effective in reducing both viral transmission and severe disease.
“Our study, utilizing a minimum of 240 million reads for each sample, was able to identify the specific gene signatures that underlie viral and interferon response. This enabled us to characterize a temporally extended and expanded genetic responses induced by a single BNT162b vaccine in the elderly with prior COVID 19 infection.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Lee, H. K., Knabl, L., Knabl, L., et al. (2021). Robust Immune Response to The BNT162b mRNA Vaccine in An Elderly Population Vaccinated 15 Months After Recovery From COVID-19. medRxiv. doi:10.1101/2021.09.08.21263284. https://www.medrxiv.org/content/10.1101/2021.09.08.21263284v1.
- Peer reviewed and published scientific report.
Lee, Hye Kyung, Ludwig Knabl, Juan I. Moliva, Ludwig Knabl, Anne P. Werner, Seyhan Boyoglu-Barnum, Sebastian Kapferer, et al. 2022. “MRNA Vaccination in Octogenarians 15 and 20 Months after Recovery from COVID-19 Elicits Robust Immune and Antibody Responses That Include Omicron.” Cell Reports 39 (2). https://doi.org/10.1016/j.celrep.2022.110680. https://www.cell.com/cell-reports/fulltext/S2211-1247(22)00432-6.