Introduction
For standard techniques in the analysis of macro-molecular interactions, such as ELISA and SPR, a binding partner is required to immobilize the molecule on a surface for the measurement of the binding affinity. For simple 1:1 association and some 1:n interactions, the equilibrium dissociation constants (KD) being quantified are not significantly affected by this physical binding. However, when both binding partners are multivalent, immobilization of one ligand can lead to the binding affinity measurements being inaccurate by orders of magnitude, caused by avidity effects at the surface, mass transport restrictions, and false assumptions regarding the stoichiometry of the interaction.
Composition-gradient multi-angle light scattering (CG-MALS) is a powerful, label-free technique that can be used to measure interactions entirely in solution, allowing binding stoichiometries to take place and enabling the measurement of multivalent interactions, such as self- and heterointeractions and metacomplex formation.
This article describes the study of complex stoichiometries arising from the association between an antistreptavidin antibody (Ab) and the homotetramer streptavidin (SA).
Instrumentation and Reagents
In the CG-MALS experiment, anti-streptavidin antibody (Amgen) and streptavidin (Sigma-Aldrich) are diluted to a final concentration of 10 µg/mL in a phosphate buffered saline consisting of PBS; 25 mM Na2HPO4, 25 mM NaH2PO4, 50 mM NaCl, 200 ppm NaN3 pH 6.7 for a total of ~100 µg protein for each experiment. Whatman Anotop syringe filters were used to filter each solution to 0.02 µm, and the resulting solutions were fed into a Wyatt Calypso® II composition-gradient system. The system prepared and supplied a variety of protein compositions and was fitted with an inline UV/Vis detector (Waters) and a Wyatt DAWN® HELEOS® MALS detector (Figure 1).
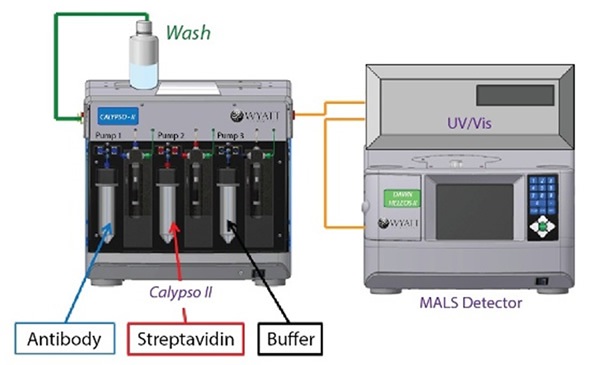
Figure 1. Calypso system hardware set-up with inline UV/Vis concentration detector and DAWN HELEOS MALS detector.
Next, the CALYPSO™ software was installed with polycarbonate (Millipore) filter membranes with 0.1 µm pore size for buffer and sample filtration. The software was used to carry out data collection and analysis of equilibrium association constants.
Results and Discussion
The intensified light scattering signal in the area of hetero-association gradient demonstrates the binding of Ab and SA to give complexes with a molecular weight greater than that of a 1:1 or 2:1 stoichiometric ratio, as shown in Figure 2.
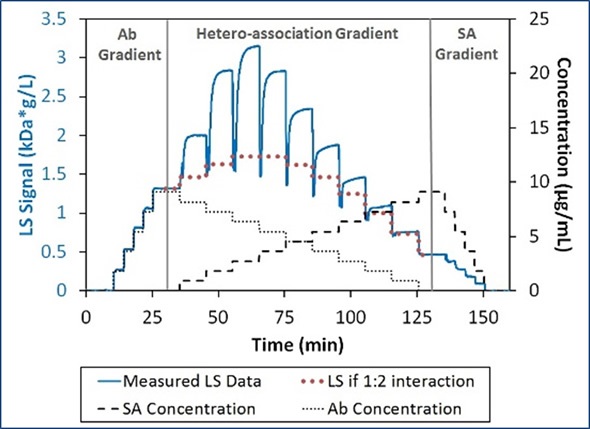
Figure 2. Light scattering and concentration data for the interaction between SA and Ab. The association is greater than can be explained by 1:2 interactions (bold dotted red line).
Under these conditions, self-association is not possible for these proteins. Therefore, the higher-order stoichiometries must be due to the multivalent nature of the two binding partners.
In a ‘crossover’ hetero-association gradient, the composition with the optimal light scattering signal (weight average molar mass, Mw) occurs at the overall stoichiometric ratio of the interaction. This ratio together with the magnitude of Mw produces the interaction’s absolute stoichiometry. Figure 3 shows the comparison of the quantified Mw to three simple SA:Ab stoichiometries.
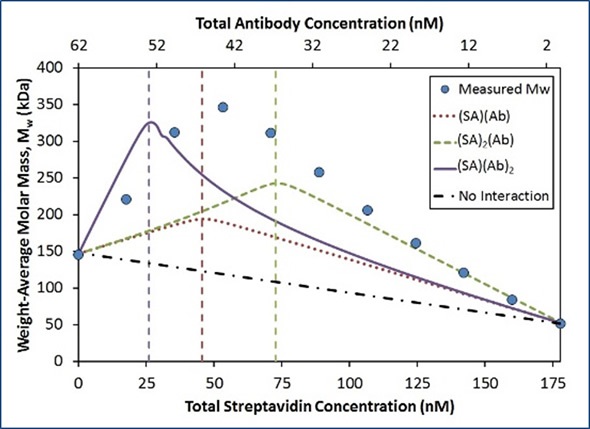
Figure 3. Measured Mw compared to simulated Mw for the indicated stoichiometries with Kd = 0.2 nM at each binding site.
The peak’s position in the quantified Mw demonstrates that the largest complexes form with a stoichiometry ratio of n:n (dotted red line) when compared with 2n:n (dashed green line) and n:2n (solid purple line). The affinity for each binding site was assumed to be constant (Kd = 0.2 nM) for each model. Although the quantified data acquired an optimum value close to the 1:1 molar ratio, the maximum measured Mw (~350 kDa) was significantly greater than the maximum molecular weight for the 1:1 model (~200 kDa).
A simulation considering two Ab bound for each SA molecule reaches the appropriate maximum Mw (~330 kDa; Figure 3, solid purple line). However, in contrast to the quantified data, this optimum value occurs at the wrong composition. This simulated model considerably underestimates the quantified Mw for all compositions that contain surplus SA.
To sum up, the data show the existence of higher order complexes with an overall stoichiometric ratio of 1:1, that is (SA)2(Ab)2, (SA)3(Ab)3, etc. This might be surprising because the comparison of the four potential binding sites on SA to the two binding sites on Ab suggests that complexes can occur with higher SA:Ab ratios, as shown in Figure 4.
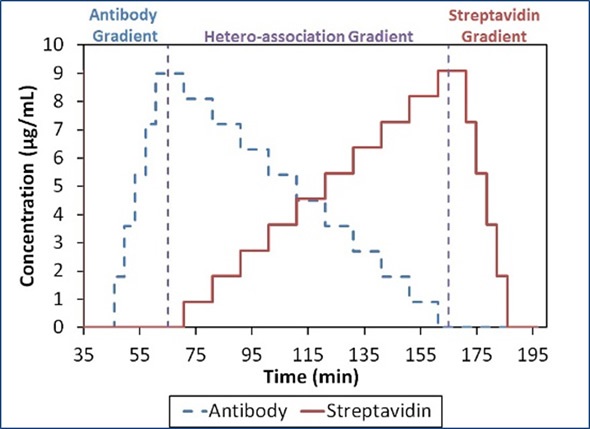
Figure 4. Composition-gradient method for quantifying the interaction between streptavidin and an anti-streptavidin antibody.
The complex which saturates the majority of the binding sites may demonstrate a total stoichiometric ratio of 2(SA):1(Ab). For the characterization of the SA-Ab interaction, two different models are considered: infinite self-association of [(SA)(Ab)] base units into n:n complexes and a more refined analysis comprising n+1:n and n:n+1 complexes as well as the n:n complexes.
First Pass Analysis
In line with this model, Ab binds to SA with an affinity (Kd) of 22 nM, and the [(SA)(Ab)] base units self-assemble with a Kd of 50 nM. To completely capture the difference in light scattering as a function of composition, an additional term representing two antibodies that bind a streptavidin molecule (SA)(Ab)2 was required. No obvious concentration of [(SA)(Ab)]n complexes is available with n>3 under these conditions, as illustrated in Figure 5.
In Figure 5, the best fit (red unfilled circles) for the measured light scattering data (blue filled circles) shows contributions from ISA, (SA) (Ab), and (SA)(Ab)2 species. The purple squares show the overall contribution to the model by all species with stoichiometry [(SA)(Ab)]n with n>1.
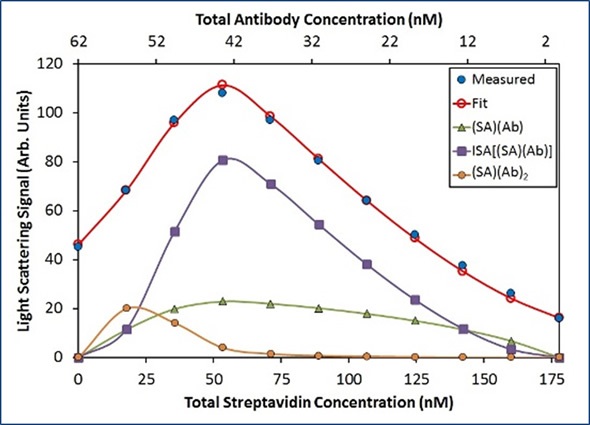
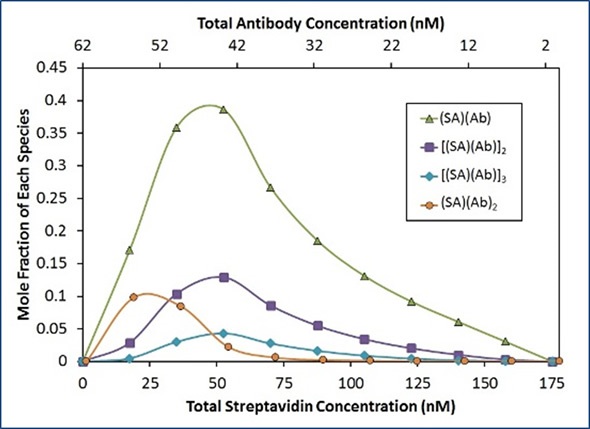
Figure 5. Best fit of CG-MALS hetero-association data using the ISA model.
Refined Analysis
Although the ISA model provides a suitable fit to the data, the model suggests that Ab attaches SA with a lower affinity if the SA molecule is already joined to another Ab. Steric hindrance may elucidate this lower affinity for complex formation. A more thorough study includes the n=1:n and n:n=1 complexes in addition to the n:n complexes.
In this analysis, (SA)2(Ab), (SA)(Ab)2, and (SA)3(Ab)2 exhibit considerable contribution to the overall light scattering, as illustrated in Figure 6. Other stoichiometries do not contribute to the total light scattering signal. This suggests that instead of the SA binding sites being saturated with Ab, it is more appropriate to saturate the Ab binding sites with SA.
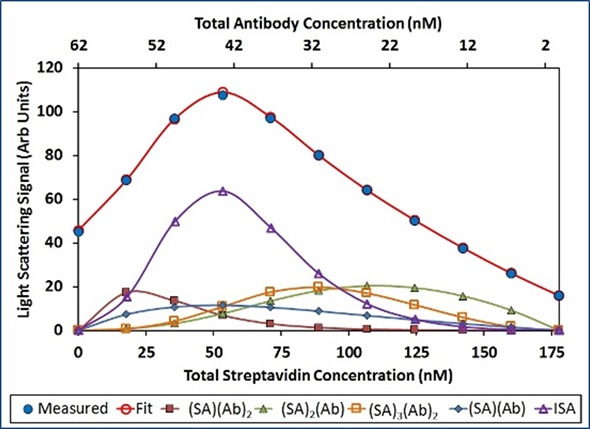
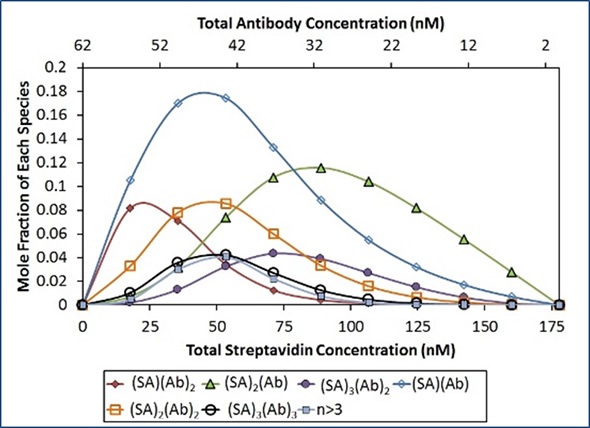
Figure 6. Best fit of CG-MALS hetero-association data assuming arbitrary (SA)i(Ab)j stoichiometries. Left: The best fit (red unfilled circles) to the measured light scattering data (blue filled circles) is made up of a combination of the indicated stoichiometries. Right: Distribution of species across the hetero-association gradient.
The constant affinity for each binding site shows the lack of negative cooperativity affecting metacomplex formation, as assumed with the ISA model. In addition, the residuals of this refined model are smaller and more random than those of the ISA model (Figure 7).
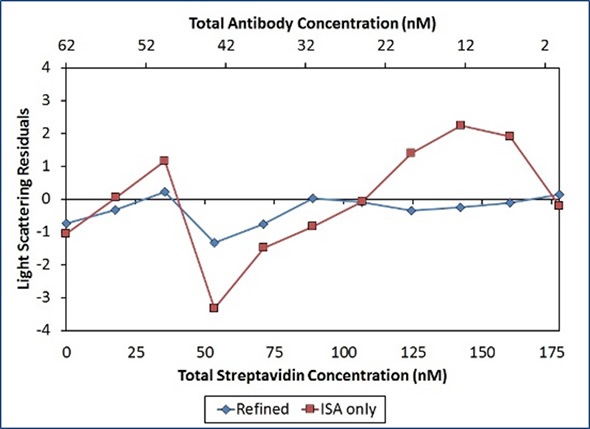
Figure 7. Residuals of the second “Refined” model (blue diamonds) are smaller and more random than residuals of the first “ISA only” model (red squares), indicating a better fit of the data.
Conclusion
The above results demonstrate that CG-MALS can analyze metacomplex formation by multivalent binding partners in solution, to obtain data on absolute stoichiometries and binding affinity. Through this analysis, CG-MALS is shown to be valuable in the analysis of complex macromolecular systems in solution.
Reference
Reprinted with permission from “Metacomplex Formation & Binding Affinity of Multivalent Binding Partners” by Wyatt Technology Corp. Graphs and illustrations reprinted with permission from Wyatt Technology.
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.