Introduction
In immunoglobulin biopharmaceuticals, aggregation is a common issue that occurs during all stages of drug development. Since a large number of monoclonal antibody titers are produced in bioreactors, effective aggregate removal techniques are required in manufacturing processes.
Aggregate Removal Capability
To this end, a high-throughput aggregate detection method could speed up assessment and validation steps in the aggregate removal process. The ability of a purification process to remove aggregates is assessed by producing and passing an IgG feed with high aggregate levels through a purification device in flow-through mode under two different buffer conditions to examine the feed sample, regeneration sample, and flow-through fractions.
Figure 1 shows the species population for sample set 1 obtained by SEC-MALS analysis. It was observed that the feed sample was composed of 45% high molecular weight (HMW) aggregates, 39% monomers and 16% dimers. Both monomer and dimer species were visible in the earlier fractions (1 to 6), while for the flow-through samples, HMW aggregates were observed in the later fractions.
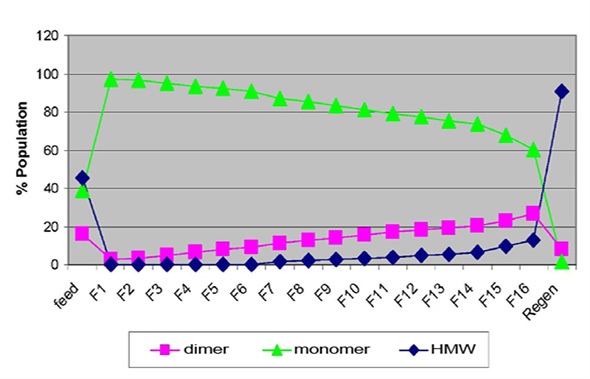
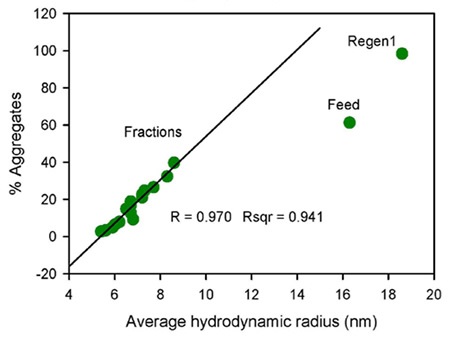
Figure 1. Flow-through profile of monomer, dimer and HMW species obtained by SEC-MALS (top image) and the correlation plot of percentage aggregates by SEC-MALS vs. average hydrodynamic radius measured by DLS (bottom image).
Results and Discussion
The regeneration peak mainly contained HMW species, which made up approximately 90% of the population. These results demonstrate that the breakthrough of non-monomer species was obvious as early as fraction 1. The dynamic light scattering (DLS) technique detected a sudden increase in average hydrodynamic radius (Rh) between fractions 1 and 2, which was consistent with the observations mentioned, as illustrated in Figure 2. This demonstrated that the early breakthrough of non-monomer species was seen in fraction 2, if not in fraction 1.
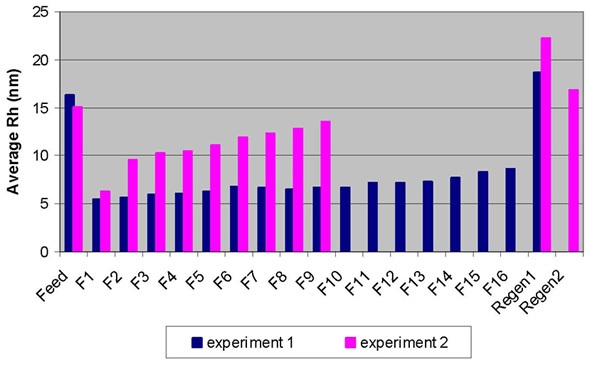
Figure 2. Breakthrough curves for Experiment 1 (in blue) and Experiment 2 (in pink) obtained by DLS.
The sudden increase in the average hydrodynamic radii between fractions 5 and 6 also suggested the immediate increase in aggregates (HMW or dimer species).
The quantified Rh value of the regeneration sample is 18.6 nm, which is greater than the value of the feed sample, at 16.3 nm, which suggest that the HMW population is higher than in the feed sample. The plotting of the percentage of aggregates obtained by SEC-MALS against the average Rh ascertained by DLS (Figure 1) showed an excellent correlation for the flow-through fractions.
Although DLS cannot provide information on species distribution (as the SEC-MALS technique can), it can be used to quickly determine the conditions that enable effective removal of aggregates from monomeric IgG. As shown in Figure 2, the operating condition in experiment 1 demonstrated enhanced separation over that of condition 2.
Conclusion
The above results demonstrate that DLS is a fast, reliable analytical technique that improves the operating conditions for aggregate removal. The time needed to complete the data acquisition for a set of 96 samples through SEC-MALS would be 32 hours, aside from sample preparation time, HPLC installation, and final data analysis. However, the installation of the DynaPro® DLS Plate Reader considerably reduced the data acquisition time and optimized Millipore’s assay capabilities.
Reference
Reprinted with permission from “Protein Aggregate Removal with the DynaPro DLS Plate Reader”, kindly submitted to Wyatt Technology Corp. by Y. Wu, Millipore, Bioprocess Division. Graphs and illustrations reprinted with permission from Wyatt Technology.
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.