Molar mass plays a vital role in the solubility, stability and potency of polysaccharide-based vaccines. The production process includes a crucial depolymerization step to cut the polysaccharide’s starting weight-average molar mass, Mw, from more than 1800 kDa down to less than 350 kDa.
Existing methods to monitor molar mass during polysaccharide production depend on off-line SEC-MALS analysis, during which one run may take up to half an hour. Normally with a depolymerization time of around 90 minutes, off-line analytics cannot deliver timely feedback on reaching the endpoint. Real-time multi-angle light scattering (RT-MALS) meets this requirement and guarantees that the process finishes when the endpoint criterion is realized.

Image Credit:Shutterstock.com
Materials and Methods
A polysaccharide solution was depolymerized by ultrasonication at 20 kHz. Molar mass was monitored online, almost in real-time, by continuously pumping a small proportion of solution from the reactor through an ultraDAWN™ RT-MALS instrument by using an Agilent HPLC pump. In order to reduce viscosity for flow through capillary tubing and to facilitate accurate measurement, the solution was continually diluted by a factor of 10.
The final flow rate was 5 mL/min, resulting in a lag time (RTD, residence time delay) of just three minutes between the time product was withdrawn from the reactor and the moment of measurement. OBSERVER™ software was set up to control the HPLC pump, gather data from the ultraDAWN™, calculate Mw 30 times per minute, and send a trigger to stop the sonication when Mw < 350 kDa, as outlined by the release specifications.
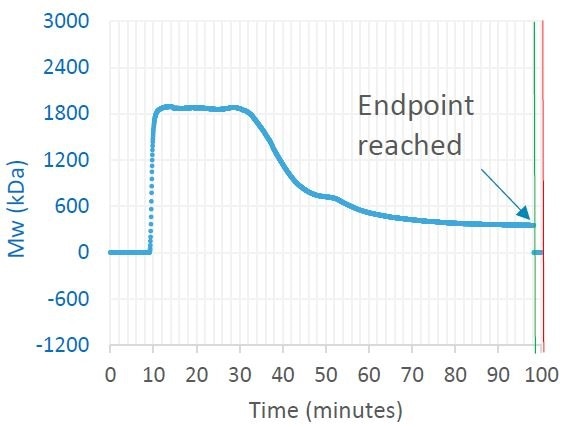
Figure 1. Trace in OBSERVER software of weight-average molar mass measured by ultraDAWN, indicating the achievement of the desired process endpoint. Image Credit: Wyatt Technology
Results and Discussion
RT-MALS noticeably tracked reduction of the polysaccharide’s Mw, and reaction shutdown was triggered once it fell below 350 kDa. SEC-MALS analysis of the final product verified the anticipated critical quality attribute (CQA) value. A number of off-line analyses were removed, the time spent by the drug substance in this part of the process was cut by 25%, and total human hours employed in this piece of the process dropped by at least 50%.
Conclusions
Polysaccharide antigens are high-value-add products. Application of PAT to monitor quality attributes provides considerable cost benefits which are obtained by in-process determination of the primary attribute impacted by the specific process. Here, the objective of the depolymerization process is to modify the molecular weight of the polymer, so determining the molecular weight in near-real-time via RT-MALS reduces uncertainties caused by process drift or disparities in raw material properties, resulting in flawless depolymerization with every run and notable cost savings.
Image Credit: Wyatt Technology
About Wyatt Technology
Wyatt Technology Corporation develops instrumentation, software and techniques for the characterization of macromolecules and nanoparticles, in solution, based on light scattering and related technologies. The physical properties determined by Wyatt’s products include absolute molar mass of proteins, polymers and other macromolecules; size and charge (zeta potential); protein-protein and other biomolecular interactions; composition of conjugated proteins and co-polymers; and macromolecular conformation.
Products and Services
Wyatt’s product line includes instruments and software for:
- on-line multi-angle light scattering (MALS), used in conjunction with size-exclusion chromatography to quantify absolute molar mass, size, conformation, conjugation and aggregation
- traditional (cuvette-based) and high-throughput (microwell plate-based) dynamic and static light scattering (DLS/SLS) to determine size and size distributions, thermal degradation and other stability-indicating parameters
- electrophoretic mobility (PALS) to determine molecular charge/zeta potential
- composition-gradient light scattering (CG-MALS) for label-free analysis of biomolecular interactions
- field-flow fractionation (AF4) for separation of macromolecules and nanoparticles from 1-1000 nm, used in conjunction with on-line MALS, DLS and other detection technologies to quantify molar mass, size, conformation and composition
Wyatt also offers, on a limited basis, sample analysis services utilizing its unique technologies.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.