The lipid nanoparticle (LNP)-based delivery of pharmaceuticals has abundant promise for novel gene therapies, oncology, and effective, fast-response vaccines. Pfizer™ /BioNTech™ and Moderna™ have created two successful examples of COVID messenger RNA (mRNA) vaccines.
Formulation to characterization
The primary components of LNP–RNA are ionizable lipids, cholesterol, PEGylated lipids, and phospholipids, which together form the nanoparticle, along with the active pharmaceutical ingredient, RNA. The surface of the LNP may be coated with targeting proteins, such as antibodies or their fragments, to ensure the payload is delivered to the correct cell type.
A common way to produce LNPs involves mixing RNA (dissolved in an aqueous solution) with the lipid mixture (dissolved in an organic solvent, e.g., ethanol). The addition of the aqueous solvent makes the lipids phase separate and results in the formation of lipid nanoparticles which electrostatically encapsulate RNA.
This process is thought to become more efficient and better controlled through rapid microfluidic mixing.
Some of the main biophysical attributes of the intermediate and final formulation products comprise particle size, polydispersity, zeta potential, and particle concentration.
The specific lipids used, as well as mixing parameters, such as solution ratio and flow rate, also affect the outcome. Because these attributes affect biodistribution and cellular uptake in addition to dosing, they are considered vital for efficacy and safety and must be characterized.
Image Credit: Waters | Wyatt Technology
Such attributes are usually measured by dynamic (DLS), electrophoretic (ELS), and static light scattering (SLS). Though some instruments incorporate all three measurement types, performing a full set on a single LNP–RNA sample is usually slow and cumbersome.
In particular, the zeta potential of LNP–RNA is especially difficult to measure accurately and repeatably when sample quantities are restricted, such as in the early developmental stages.
DynaPro ZetaStar
The DynaPro ZetaStar DLS/ELS/SLS instrument by Wyatt Technology enables fast, streamlined characterization of LNP–RNA.
With the micro-cuvette and dip cell, ELS, DLS, and SLS can be measured for size, polydispersity, zeta potential, and particle concentration in a single workflow using just 65 µL of sample - a process that takes under a minute.
The Dynapro ZetaStar instrument seamlessly integrates an Arc pump and autosampler, making it fully compatible with automated workflows. Size, polydispersity, and zeta potential can be calculated measured for an entire series of formulation conditions ,which requires about 500 µL per sample.

Figure 1. The DynaPro ZetaStar DLS/ELS/SLS instrument features walkup operation with an intuitive touch-screen application, DYNAMICS Touch™, that guides users through the measurement process. It can be operated manually, in microcuvette mode, or combined with an autosampler for automated analysis of many samples in DYNAMICST™ software. Image Credit: Waters | Wyatt Technology
Instead of using the same photodetector for each measurement type, ZetaStar incorporates separate detection channels that are optimized for each technique, enabling parallel measurements of DLS and SLS (size, polydispersity, particle concentration) or DLS and ELS (size, polydispersity, zeta potential) in the shortest possible amount of time.
The determination of the zeta potential of LNP–RNA in a solution that contains appreciable ionic strength (more than about 10 mM NaCl) has been a frustrating issue regarding most ELS instruments.
ELS relies on the application of an electric field under which the charged analyte particles move at a velocity relative to their zeta potential.
High applied voltage allows fast measurement but can degrade particles, leading to unreliable results. Conversely, low applied voltage leads to a weak response and necessitates long measurement times.
The ZetaStar instrument implements an advanced electro-optical technique that enables highly sensitive detection, even at low-applied electric fields.
This technique, also known as fiber interferometric doppler electrophoretic light-scattering (FIDELIS), offers fast and reliable zeta potential determination while simultaneously measuring size and polydispersity in the DLS channel, as shown below.
ZetaStar can be operated in walk-up mode through the instinctive DYNAMICS Touch onboard app or with full 21CFR Part 11 compliance through the comprehensive DYNAMICS software on a host PC.
By quantifying numerous quality attributes on a single instrument, ZetaStar contributes to pharmaceutical studies of dosing, stability, formulation, and lot-to-lot variability across process and product development cycles, as well as quality control in product manufacture.
Materials and methods
LNPs (University of Pennsylvania) were prepared either by microfluidic or hand mixing. Before measurement, the neat LNPs were diluted 100-fold in 0.02 µm-filtered 20 mM tris buffer (pH 7.4).
The samples were an empty LNP prepared via microfluidic mixing (S1), an LNP loaded with mRNA and prepared by microfluidic mixing (S2) and an LNP loaded with mRNA and prepared via hand mixing (S3).
Batch DLS, ELS, and SLS measurements were implemented in a dip cell with ZetaStar, and data acquisition and analysis were executed via the DYNAMICS software. An adaptive collection mode was enabled for the optimization of the applied current and measurement time, which resulted in an average of one minute per sample.
Results and discussion
Size and distribution
DLS measurements exposed substantial differences in the size distribution for the LNPs prepared by microfluidic compared to hand mixing. Figure 2 shows results for the size distribution of all three LNPs.
The empty LNP prepared using microfluidic mixing (S1) seems to be the smallest of the three, with a mean radius of around 30 nm and a small amount of aggregate (around two hundred nanometers).
Using the same technique to create mRNA-loaded LNP produced particles with an average radius of around 40 nm and a single broad-sized distribution (S2). These distributions agree with predictions and are within the anticipated range for optimal circulation and cellular uptake.
In addition to the presence of nucleic acid influencing the size of LNPs, the processing parameters used for generating LNPs (or even the process itself) can substantially impact the size of the final production particles.
As shown in Figure 2, the hand-mixing process (S3) produces significantly larger LNPs with comparable polydispersity. The average radius of these LNPs was nearly twice as large as the LNPs created using the microfluidic mixing approach. Such a size may be undesirable for bioavailability and functionality.
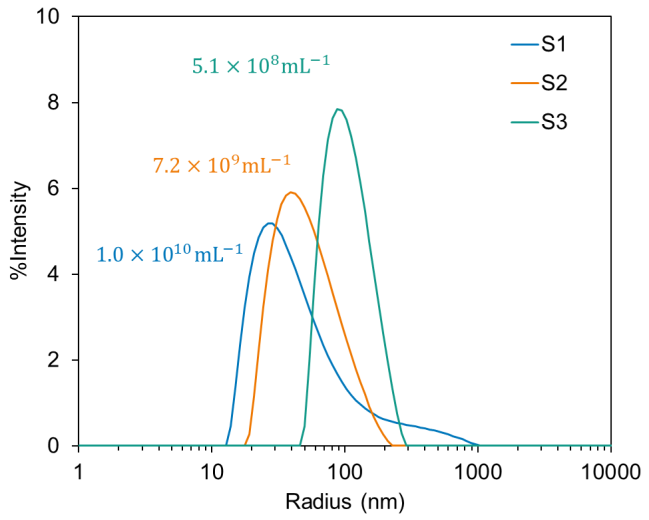
Figure 2. Size, size distribution, and concentration of empty LNP prepared via microfluidic-mixing (S1), LNP with mRNA prepared via microfluidic-mixing (S2), LNP with mRNA prepared via hand-mixing (S3). Image Credit: Waters | Wyatt Technology
Concentration of particles
The concentration of particles reveals yield information and informs dosing. The dedicated SLS detector facilitates measurement of particle concentration in under a minute, without reagents or particle concentration standards.
ZetaStar enables simultaneous size and particle concentration measurement in quantities as low as 2 µL with a quartz cuvette. The concentration results from three LNPs can be found in Figure 2 (summarized in Table 1). Such data suggests that the hand-mixing procedure generates fewer larger particles, while microfluidic mixing produces a higher number of smaller particles.
Zeta potential
The ZetaStar instrument can perform simultaneous DLS and ELS to determine size and zeta potential. The zeta potential analysis (Figure 3) shows that all three LNPs display a consistent negative charge regardless of their preparation method or whether they are loaded or empty.
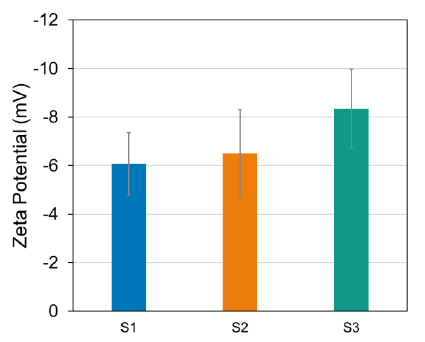
Figure 3. Zeta potential of empty LNP prepared via microfluidic-mixing (S1), LNP with mRNA prepared via microfluidic-mixing (S2), LNP with mRNA prepared via hand-mixing (S3). Image Credit: Waters | Wyatt Technology
These results are also summarized in Table 1. It must be noted that the zeta potential is a measure of the surface charge of the nanoparticles; it does not include the charge of buried mRNA. In this case, the presence of the negatively charged mRNA inside the LNP did not significantly change the particle's net surface charge.
Table 1. Average hydrodynamic radius, zeta potential, and particle concentration of LNP samples. Source: Waters | Wyatt Technology
| LNP condition |
Radius (nm) |
Zeta potential (mV) |
Particle concentration (mL-1) |
| S1: Empty, microfluidic mixing |
38 ± 2 |
-6.1 ± 1.3 |
(1.0 ± 0.3) x 1010 |
| S2: Loaded, microfluidic mix-ing |
44 ± 1 |
-6.5 ± 1.8 |
(7.2 ± 1.1) x 109 |
| S3: Loaded, hand mixing |
98 ± 2 |
-8.4 ± 1.6 |
(5.1 ± 0.5) x 108 |
| All values are average and standard deviation of 10 measurements. |
Measuring LNP-RNA size, size distribution, zeta potential, and particle concentration with the DynaPro ZetaStar instrument offers a streamlined approach for rapid screening of LNP formulations and process conditions. The ZetaStar is a simple, powerful, and versatile tool that not only measures size, polydispersity, zeta potential, and particle concentration for nanoparticles but also determines molar mass, turbidity, interactions, and thermal behavior for macromolecules like polymers, proteins, and nucleic acids.
While the ZetaStar is ideal for quick screening, detailed characterization of LNP-RNA, including particle size distributions, concentration, encapsulation efficiency, and size-based payload, can be further enhanced with separation-based techniques such as SEC-MALS and FFF-MALS.
Acknowledgments
Produced from materials originally authored by Xujun Zhang, Ph.D., and Sophia Kenrick, Ph.D., from Wyatt Technology, LLC.
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20 % Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20 % Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.