Introduction
Antibody-drug conjugates (ADCs) are a new type of drug that are currently the subject of much interest due to their potential application in the targeted delivery of cancer treatments. One of the key factors in the effective design of ADCs is the Drug Antibody Ratio (DAR). This article describes a method of DAR determination based on SEC-MALS coupled with differential refractive index detection and UV absorption.
DAR Determination in the Development of ADC Therapeutics
DAR defines the amount of the appended drug that directly influences its potency and potential toxicity and can have substantial effects on characteristics such as stability and aggregation. Therefore, it is essential to determine DAR in the development of novel ADC therapeutics.
Disadvantages in Current Drug-Antibody Component Analysis
Mass spectrometry (MALDI-TOF or ESI-MS) or UV spectroscopy is generally used to determine DAR. Calculations based on UV absorption are often difficult due to similarity between the extinction coefficients of the small molecule and the antibody. While mass spectrometry is a powerful technique for determining weight average molar mass (Mw), it requires uniform ionization and recovery between compounds and this does not always exist for ADCs.
ASTRA 6 Software Tool
Figure 1 shows the UV traces for two model ADCs. Molecular weights of the entire ADC complexes are measured directly from light scattering data. Component analysis is then programmed within the ASTRA® 6 software package using extinction coefficients and differential refractive index increments (dn/dc), which are either empirically calculated for each species or obtained from the literature. This enables the molar mass to be calculated for each component of the complex and for the entire ADC complex. Here, antibody is alkylated with a compound that has a nominal molecular weight of 1250 Da, as shown in Figure 2.
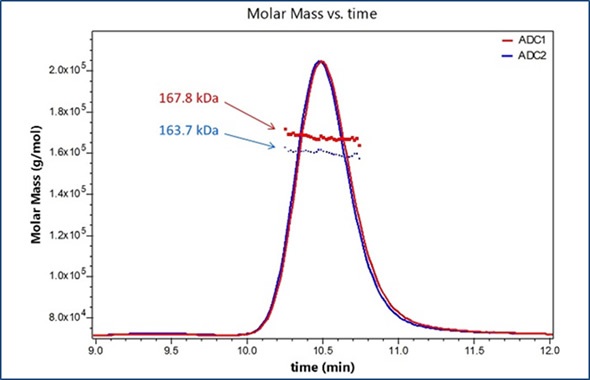
Figure 1. Molar masses for two distinct ADC formulations are determined using SEC-MALS analysis.
|
|
Mw (kDa)
|
|
| |
Complex
|
Antibody
|
Drug
|
DAR
|
|
ADC1
|
167.8 (± 1.2%)
|
155.2 (± 1.8%)
|
12.6
|
10.1
|
|
ADC2
|
163.7 (± 1.2%)
|
155.6 (± 1.2%)
|
8.1
|
6.5
|
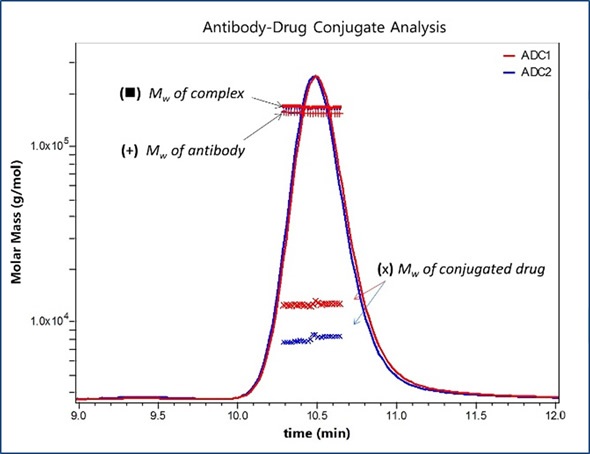
Figure 2. Molar Masses for the antibody and total appended drug are calculated in the ASTRA software package based on prior knowledge of each component’s extinction coefficient and dn/dc, allowing determination of DAR based on a nominal Mw of 1250 Da for an individual drug.
Similar molar masses are exhibited by all antibody fractions, meaning that the overall variations between the two formulations show distinct average DARs that correspond to the values obtained through orthogonal methods. The amount of attached pendant groups is denoted by the molar mass traces for the conjugated moiety, whilst the horizontal trends denote that change is uniform across the population eluting in that peak.
Conclusion
Conventional SEC relies on reference standards that do not accurately represent the molecules under study. In contrast, SEC-MALS is an established analytical method that can directly measure the molar mass of large molecules. This approach is suitable for many types of molecules such as proteins, peptides and polymers.
Reference
Reprinted with permission from “Antibody Drug Conjugate (ADC) Analysis” by Wyatt Technology Corp. Graphs and illustrations reprinted with permission from Wyatt Technology.
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.