A non-aggregated sample is vital for small-angle neutron scattering (SANS). The utilization of the DynaPro dynamic light-scattering instrument has substantially assisted studies elucidating the low-resolution shape of numerous Type I bacterial restriction-modification systems.
Although the protein samples were deemed acceptable by UV-spec and SDS-PAGE (judged by the flat baseline and low minima at 260 nm), some of the SANS measurements showed that some of the protein samples were prone to aggregation, and thus, poor measurements were attained.
After purification, dynamic light scattering (DLS) was then used to access the monodispersity of the protein samples.

Figure 1. The DynaProTM NanoStarTM is a cuvette-based DLS instrument with a user-friendly touch interface. It allows for convenient, walk-up measurement of size, polydispersity, and particle concentration, requiring as little as 2 µL of samples. Image Credit: Waters | Wyatt Technology
Experiment
DLS was performed at 10 °C in 10 mM Tris.HCl pH 8.0, 100 mM NaCl, and 1 mM Na2EDTA with a DynaPro cuvette-based DLS instrument.
Results and discussion
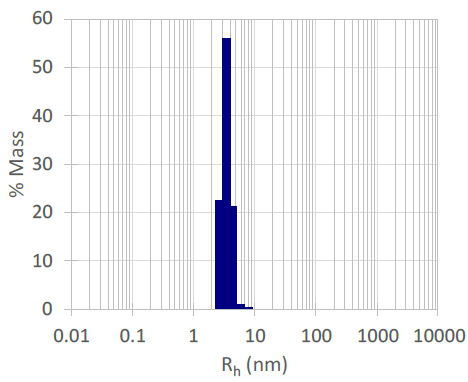
Figure 2. Histogram from the DYNAMICS software used to show the hydrodynamic radius and polydispersity of the R subunit of EcoR124I. Image Credit: Waters | Wyatt Technology
The hydrodynamic radius, Rh, and polydispersity values were obtained from 30 positive measurements using % mass calculations.
The size-based molecular weight, Mr, was established using a volume shape hydration model and compared to the theoretical Mr, which was found to be in very good agreement. The sample was non-aggregated and, therefore, suitable for SANS measurements.
This allowed the researchers to determine which proteins could be stored and those that needed to be freshly purified before complex formation. The maximum concentration of the proteins before the formation of aggregates was determined.
The highest-quality SANS data in the shortest measuring time on D22 (Institut Laue-Langevin, Grenoble) could, therefore, be calculated, allowing researchers to collect as much data as possible in this beam-time allocation.
DLS is now regularly used to monitor the behavior of the protein in solution.
Acknowledgments
Produced from materials originally authored by Dr. James E. N. Taylor, Biophysics Laboratories, School of Biological Sciences, University of Portsmouth.
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20 % Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20 % Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.