Newcells provides a quick and accurate in vitro retinal drug toxicity testing service using intricate retinal organoid models created in-house or from client-supplied cells. The retinal toxicity service assesses the impact of novel substances on cell viability after photoreceptor degeneration.
Newcells also compares multiple differentiation time points. This quick retinal toxicity service helps customers assess potential rescue tactics and gain insights into their compounds' ocular toxicity profile.
Service outputs
- Quick assessment of the toxicity of substances in iPSC-derived retinal organoids
- Qualitative immunofluorescence of key markers' expression on frozen retinal organoid sections
- Comparison of time points
- Evaluation of the makeup of Muller glia, amacrine, photoreceptors, retinal ganglion cells, and horizontal cells with imaging methods
Assays
- Gene expression
- Transmission electron microscopy (TEM) and scanning electron microscopy (SEM)
- Cell viability assays
- Key marker analysis
- Quantitative immunofluorescence
Models
- Human retinal organoids
- Originated from patient or client-provided gene-edited iPSCs
- Derived from fibroblasts or PBMCs that the client provides
- Derived from WT iPSCs
Timeline
How to access the retinal service?
Outsource the experiments to obtain robust data
Newcells creates experiments to address the user’s inquiries with quick and dependable service, utilizing its own RO or creating them from clients' patient cells. Data after the study will show the compounds’ retinal toxicity profile and on-demand rescue techniques. A group of retina specialists in cutting-edge UK facilities complete custom projects.
Generation of patient-derived retinal organoids as part of the service
The complete service includes the generation of retinal organoids from patient-derived or gene-edited iPSCs, PBMCs, or fibroblasts supplied by the client, feasibility studies, experimental design, compound testing, and data restitution for regulatory submission.
Rapid in vitro retinal safety/efficacy assessment of new drugs: retinal toxicity made easy
Some drugs, when administered systematically, may affect retina function. Similarly, new treatments for eye diseases must undergo rigorous safety testing. Animal and human ex-vivo models can be used to study retinal toxicity, but they are limited by the number of experimental data points generated and human predictivity.
Scientists at Newcells have created reliable retinal tissue models using human iPSCs. A meticulously regulated differentiation process replicating embryonic retinogenesis chronology yields retinal organoids.
By day 150, the organoids are functional and comprise all major cell types, making it possible to assess the cytotoxic effect of novel compounds by merely adding them to the plate. Next, the integrity of the cell structure and the gene expression profiles of important markers for the primary cell types—like photoreceptors—are evaluated.
Newcells also conducts microscopy and qualitative imaging to provide a thorough drug toxicity profile. The models offer important information for transitional studies and directly apply to human clinical trials because they are derived from human iPSCs. The primary benefits of in vitro retinal toxicity testing are its predictability for human clinical trials and speed, as most basic studies can be completed in one to two months.
These investigations can be conducted in vitro using retinal organoids to quickly assess the retinal toxicity of novel compounds.
Modeling retinal drug toxicity: Retinal organoids were tested for the response of known toxins like thioridazine and doxorubicin. Doxorubicin’s intrinsic fluorescence makes it easier to see how the drug penetrates the retinal organoid (A). Doxorubicin exposure reduces the viability of iPSC-derived retinal organoids in a dose-dependent manner (B).
Drug screening is a crucial step in the early stages of drug development because it helps identify potential compounds. Typically, medium to high throughput is used for this. Newcells Biotech retinal organoids are produced in a 96-well plate format every 4-6 weeks (on-demand supply), so they are appropriate for in vitro drug screening. They have been employed to differentiate between harmful and non-toxic substances in the retina.
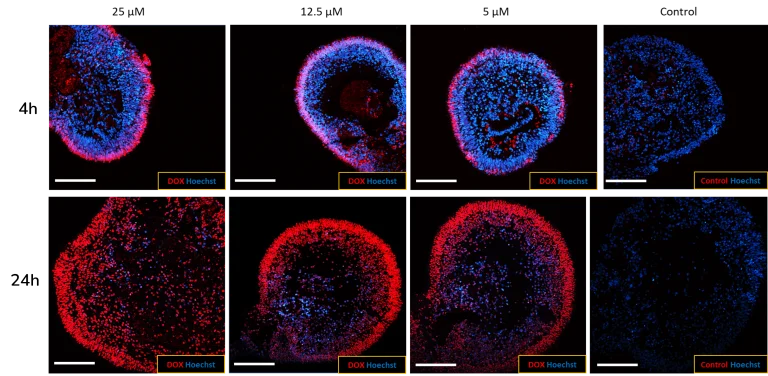
(A) Newcells’ human iPSC-derived retinal organoids are permeable to small molecules. The penetration of doxorubicin, a naturally fluorescent small toxic molecule (red), into the retinal organoids, increases over time (4 h to 24 h), demonstrating the permeability of the organoids to drugs. Image Credit: Newcells Biotech
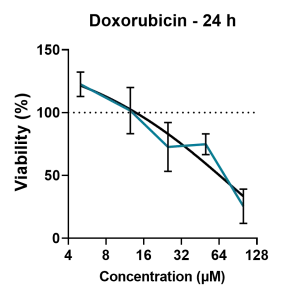
(B) The retinal organoids were treated with increasing doses of doxorubicin over a period of 24 h, and cell viability was measured using an ATP assay. A dose-dependent decrease in cell viability was observed following increasing exposure to the drug. Image Credit: Newcells Biotech
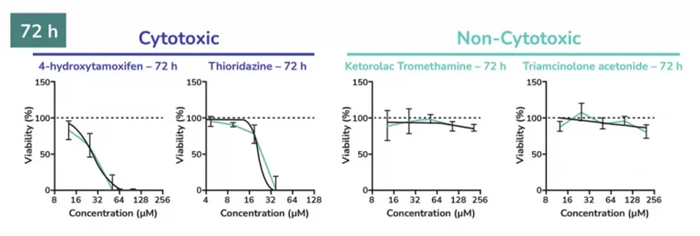
Dose-response plots of known cytotoxic and non-cytotoxic retinal agents. Retinal organoids were exposed to increasing concentrations of either cytotoxic or non-cytotoxic drugs, and cell viability was measured over time. As expected, cell viability decreased upon increasing the addition of Thioridazine and 4-hydrohytamoxifen, while non-cytotoxic drugs had no effect. The dose response of cytotoxic and non-cytotoxic retinal agents was determined using CellTiter-Glo® 3D ATP assay. Image Credit: Newcells Biotech
Service overview
Newcells offers a customized service to assess retinal toxicity using the complex iPSC-derived human retinal models (retinal organoids).
Although retinal organoids can take up to 210 days to differentiate, the streamlined manufacturing process produces a consistent supply of tissue, allowing users to meet short project timelines. The scientific experts’ robust data will give users confidence as they make key decisions during drug development.
One example of retinal drug toxicity screening includes a set of assays for cell viability, photoreceptor functionality, degeneration, key marker expression, and localization. The safety of novel viral vectors, such as AAV, can also be assessed.
Source: Newcells Biotech
| Assay design |
| Models |
Retinal organoids with photoreceptors (cone and rod), retinal ganglion cells, horizontal cells, and amacrine cells (WT). |
| Assay format |
96-well plates (retinal organoids) |
| Species |
Human |
| Assay readout |
Cell viability assay (ATP depletion assay, LDH release and microscopy) |
| Qualitative immunofluorescence with cell-specific markers & apoptosis markers |
| Gene expression profile of key marker gene by RT-qPCR |
| Microscopy: 2D-TEM and SEM |
| Time points and replicates |
Retinal organoid toxicity service can be performed at any time point of differentiation up to Day 210. |
| Data points are usually performed in triplicates or quadruples with a minimum of 10 organoids. |
Models to choose from for this service
Retinal organoids
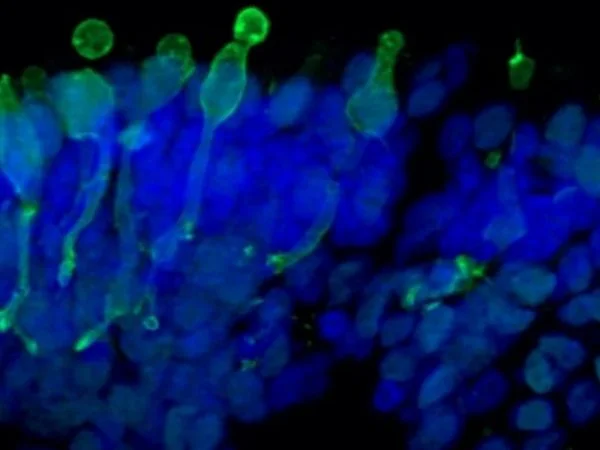
Cone photoreceptor cells labeled with anti-Opsin (Red/Green) antibodies. Image Credit: Newcells Biotech
The retinal organoids are iPSC-derived and replicate the complex structure of the human retina, including laminar cell organization that mimics embryonic development. They contain the retina's outer photoreceptor segment, which responds to light.