Newcells' retinal organoids and RPE models are effective tools for preclinical in vitro testing of new retinal gene therapy vectors. They enable quick evaluation of the best vector capsid, promoter, and transgene combinations.
These models also support early safety and efficacy testing using the same promoter or gene planned for clinical trials. Newcells' retinal models have been validated for screening and selecting the most effective adeno-associated viral (AAV) vectors with enhanced photoreceptor targeting for retinal gene therapy.
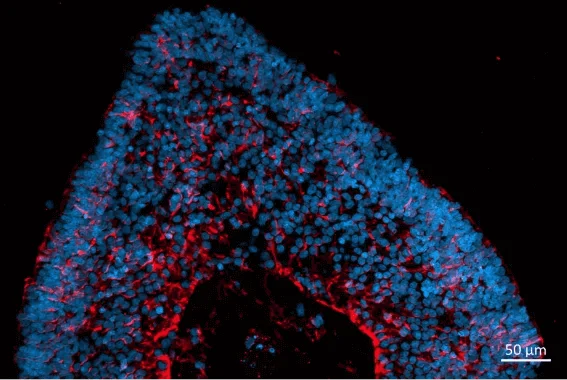
Localization and distribution of Müller glia cells (CRALBP, red) in retinal organoids. Nuclear DAPI staining (blue). Image Credit: Newcells Biotech
Service outputs
- Evaluation of AAV vector transduction efficiency in iPSC-derived retinal organoids or retinal pigment epithelial (RPE) cells
- Assessment of AAV vector tropism and transduction of photoreceptor-like cells in retinal organoids
- Cell viability testing after transduction
Outputs
- Therapeutic efficacy
- Cell tropism identification in retinal organoids
- Transduction efficiency of viral vector
- In vitro safety prediction
- Cell viability assessment
Models
- Human retinal organoids
- Human RPE cells
- Obtained from the CRISPR/Cas9 gene or the patient-edited iPSCs provided by the client
- Produced from patient or client-supplied PBMCs or fibroblasts with the CRISPR/Cas9 gene altered
- Derived from healthy donor
Timeline
How to use gene therapy vector assessment service?
Newcells' team works with customers to design experiments to help them choose the best gene therapy vector for target cells. A dependable and quick service makes rapid in vitro screening of novel viral vectors in a human model possible. The data supports IND applications and has been validated. All custom projects are completed in modern UK facilities.
Take advantage of the full service, which includes creating organoids and RPE from patient-derived or gene-edited iPSCs, PBMCs, or fibroblasts, conducting feasibility studies, designing experiments, testing viral vectors, and producing accurate data for regulatory submissions.
AAV vector evaluation in vitro for retinal gene therapy
Retinal organoids from Newcells have demonstrated the ability to serve as an instructive in vitro model for assessing novel retinal gene therapy vectors for retinal diseases.
Organoids have been utilized to screen AAV gene therapy vectors in cooperation with a group at the University of Oxford. This study demonstrated the enhanced transduction capability of AAV2 7m8 in retinal organoids when employing the ubiquitous CAG promoter, confirming robust and efficient transduction of human photoreceptor-like cells by AAV vectors.
The experiments demonstrated that a CAG-driven transgene transduced various cell types, whereas GRK1-driven transgenes exhibit a more limited photoreceptor-specific expression (A). This was because the organoids contained multiple cell types, which allowed the experiments to display the targeted cell types specifically. The study also showed that AAV transduction did not affect the retinal organoids’ viability (B).
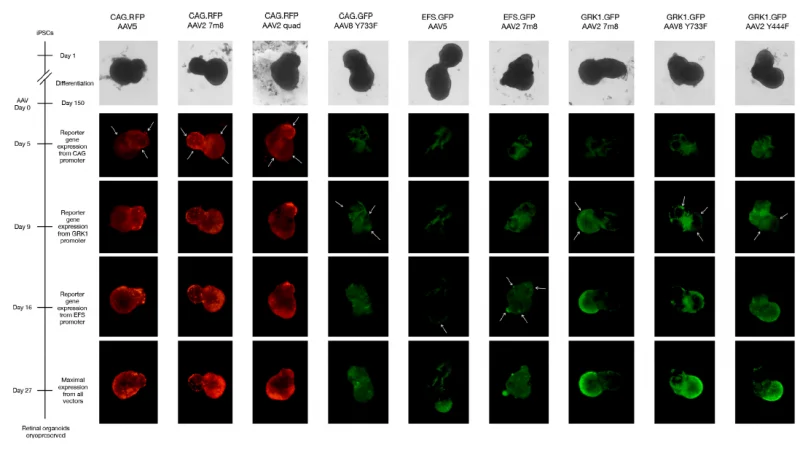
(A) Live cell imaging of reporter gene expression up to 27 days post-transduction. Arrows indicate the areas where the onset of reporter gene expression first appeared, allowing evaluation of which cell types each AAV vector transduced preferentially. Image Credit: Newcells Biotech
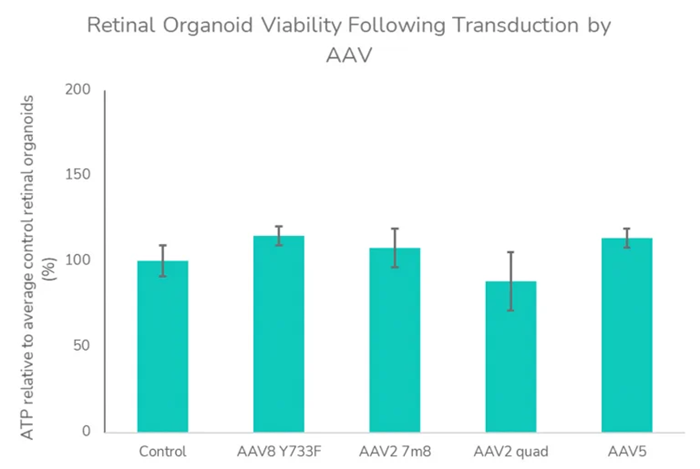
(B) Retinal organoid viability following transduction by AAV. ATP assay was assessed as an indicator of viability at 27 days post-transduction with AAV vectors. Data analyzed using a one-way analysis of variance indicated no significant influence of AAV treatment on ATP levels and viability (F=1.107, P =0.3908) relative to control retinal organoids. Image To enable quick assessment of novel retinal gene therapy vectors, these investigations can be conducted in both retinal organoids and RPE in vitro models. Image Credit: Newcells Biotech
These investigations can be conducted on Newcells' retinal organoids and RPE in vitro models to enable quick assessment of novel retinal gene therapy vectors.
Transduction efficiency in RPE
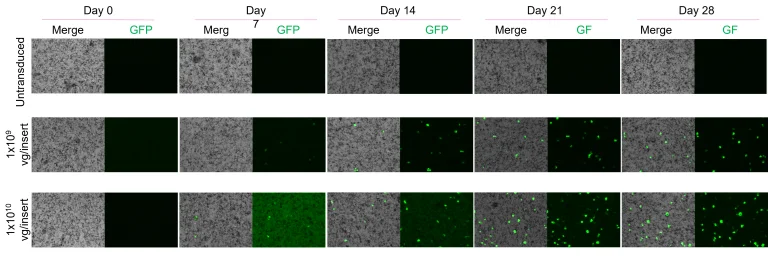
Dose- and time-dependent increase in GFP signal in AAV-transduced RPE. Percentage of GFP-positive cells over 28 days post-transduction with AAV5-CAG-EGFP:WPR at two different doses. Scale: 150 µm. Image Credit: Newcells Biotech
Service overview
AAV vector evaluation in labs is a custom service offered by Newcells. The project’s timelines are short as tissue is regularly available. During the crucial decision-making stages of drug development, users will be confidently guided by the solid data produced by the scientific experts.
A collection of tests to evaluate post-transduction cell viability, cell tropism, and transduction efficiency is an example of a viral vector testing service.
Assay Design. Source: Newcells Biotech
| Assay Design |
| Models |
Retinal organoids with photoreceptors (cone and rod), retinal ganglion cells, horizontal cells, and amacrine cells. |
| Retina pigment epithelium (RPE) cells (2D cobblestones monolayer). |
| Assay format |
96-well plates (retinal organoids) |
| 24-well plates (RPE) |
| Species |
Human |
| Assay readout |
Cell viability assay (ATP depletion assay, LDH release and microscopy) |
| Qualitative immunofluorescence with cell-specific markers & cell death markers |
| AAV transduction efficiency |
| Therapeutic efficacy |
| Time points and replicates |
Post-transduction (typically up to 28 days) |
| Triplicates per vector and per concentration |
Models to choose from for this service
Retinal organoids
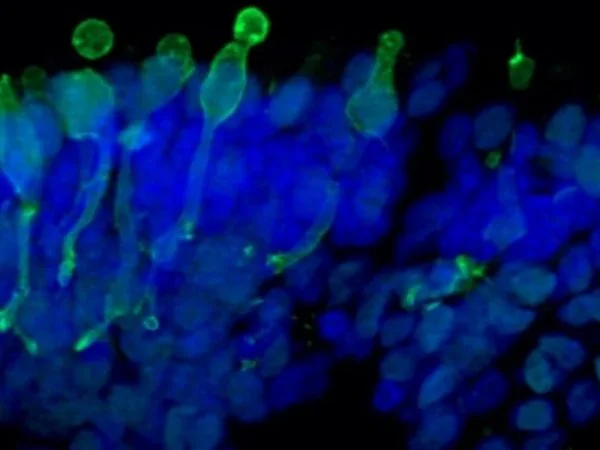
Cone photoreceptor cells labeled with anti-Opsin (Red/Green) antibody. Image Credit: Newcells Biotech
With a laminar cell organization resembling embryonic development, the retinal organoids, derived from iPSCs, replicate the intricate structure of the human retina. They house the retina’s light-responsive outer photoreceptor segment.
Retinal pigment epithelium cells
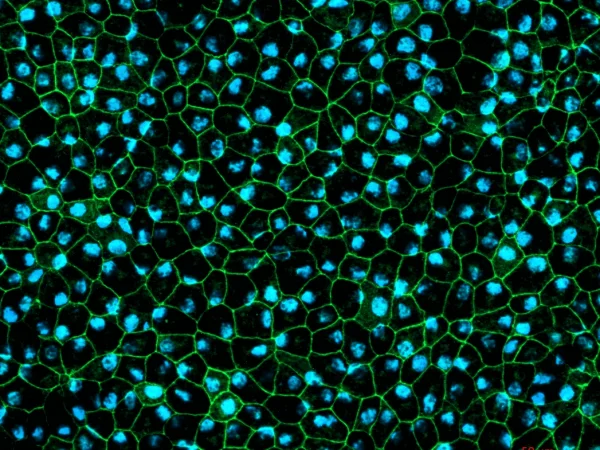
RPE cells displaying cobblestone morphology. Cells were immunolabeled with tight-junction ZO-1 marker (shown in green) and co-stained with nuclei marker, Hoechst (shown in blue). Image Credit: Newcells Biotech
A two-dimensional in vitro model of retinal pigment epithelial cells derived from human induced pluripotent stem cells that replicate the phagocytosis of outer segments of photoreceptors. The RPE cells exhibit the characteristic cobblestone morphology and are pigmented.