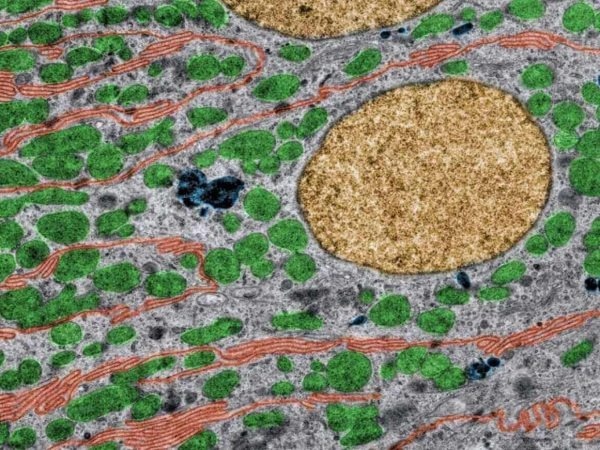
Colored Transmission electron microscopy (TEM) of kidney tubule cells. Image Credit: Newcells Biotech
While new drug molecules are typically tested in vivo in two preclinical species to better understand how they are transported and eliminated by the kidney and assess the risk of renal toxicity in humans, these animal models do not always accurately predict human responses.
To overcome this challenge, robust in vitro kidney transporter models like aProximateTM are valuable tools for comparing species and improving in vitro-in vivo extrapolation.
Assays
- Drug transporter assays and DDI
- Flux and net flux drug transporter assay
- Uptake assays: Intracellular drug and metabolite concentrations
- High content imaging
Model
- aProximateTM kidney proximal tubule cells
Species
How to use aProximateTM for the assessment of drug transporter interactions
Customers in the USA and Europe can order aProximateTM assay-ready plates from Newcells. This allows for the utilization of the recently isolated proximal tubule cells in the laboratory setting to better understand possible drug interactions.
Human proximal tubule cells are available as assay-ready plates (rat launching soon). Upon receipt, the model comes with a maintenance medium and user manual that includes a thorough protocol for cell recovery.
The consistent supply of fresh kidney tissue allows Newcells to provide prompt and dependable service, creating experiments that fully address drug-handling inquiries. The data will offer important insights into the compounds' pharmacokinetic and ADME profiles for efficient lead optimization. The data has been utilized for regulatory filings. The team of kidney specialists completes the custom projects in cutting-edge UK facilities.
Example 1: Cross-species comparison of drug handling of PAH excretion in the kidney transporter model
Para-aminohippurate (PAH), a derivative of hippuric acid, is used to measure renal plasma flow. When administered intravenously, PAH is primarily extracted from the blood by OAT1, the key transporter for PAH. Adding probenecid, an OAT1 inhibitor, reduces PAH clearance due to drug-drug interactions at the transporter level.
Using aProximateTM PTCs from four distinct species, PAH excretion was assessed in vitro to see if any variations in PAH handling could be identified. Human, non-human primates, dogs, and rat PTCs all had net secretions of PAH, according to measurements of PAH apical to basolateral flux (Jab), basolateral to apical flux (Jba), and net flux (Jnet). aProximate™ is the best kidney transporter model to test drug handling and safety in various species in vitro before choosing preclinical species.
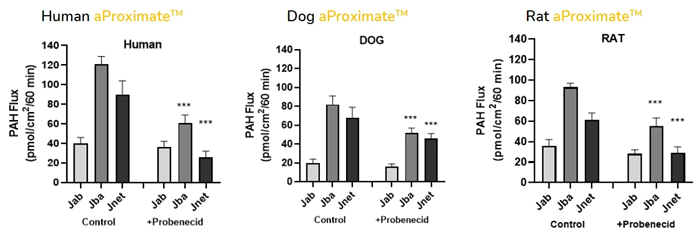
Cross-species comparison of PAH drug handling in human, dog, and rat aProximate™ proximal tubule cells. Apical to basolateral flux (JAB), basolateral to apical flux (JBA), and net flux (JNet) suggest a net secretion of PAH in human, dog, and rat aProximate™. Image Credit: Newcells Biotech
Example 2: Understanding the molecular basis of renal toxicology differences of a compound using a kidney transporter model
As part of a collaborative project, Newcells has examined renal toxicology after exposure to the herbicide MCPA (4-chloro-2-methylphenoxyacetic acid) to clarify the molecular basis of species differences observed (Gledhill et al., 2022).
MCPA is a globally registered herbicide for controlling broadleaf weeds in cereals. Toxicological studies with MCPA revealed that the kidneys were the primary target organ after oral dosing in rats, mice, and dogs. Toxicokinetics of MCPA and other derivatives in rodent and non-rodent models revealed that kinetic differences in dogs indicate this species is less relevant to human risk assessment than rodents.
This study aimed to determine the molecular basis for the dog's increased sensitivity to the toxic effects of MCPA compared to humans and rats. The work included in vitro renal transporter studies using Newcells' aProximateTM model, comparing proximal tubule cell flux in rats, dogs, and humans.
The data was analyzed to provide a mechanistic rationale for the higher systemic exposures in dogs compared to other species and to support the dog's lack of relevance as a model species for MCPA human health risk assessments.
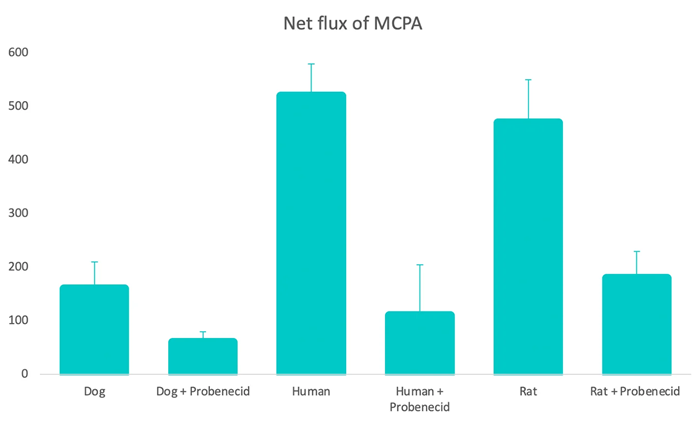
Net flux of MCPA (in ng/cm2/hr) through renal proximal tubule cell monolayers from rat, dog, or human donors. MCPA (50 μg/ml) incubated for 90 min in the presence or absence of OAT1 inhibitor probenecid (200 μM). Image Credit: Gledhill et al., (2022)
Service overview
The service offers insights into cross-species differences in drug handling. aProximate™ is available in multiple species, such as human, rat, dog, and NHP, making it the ideal kidney transporter model for assessing cross-species drug handling.
Using the transporter assays, quickly compare species in vitro to choose the most promising preclinical species and advance lead compounds into clinical development.
For IND and IMPD submissions, the cross-species comparative data produced by the transporter assays has been utilized since it offers comprehensive mechanistic insights into drug excretion in multiple species, elucidating potential variations.
The consistent supply of fresh kidney tissue allows users to complete projects on short timelines. With the help of solid data produced by scientific experts, users can make confident decisions about important aspects of drug development.
Evaluating transepithelial flux in proximal tubule cells, particularly Apical to Basal (Jab) and Basal to Apical (Jba) flux, and net transport measurements in rats, dogs, and humans, are examples of cross-species drug interaction packages. The amount of intracellular accumulation across the apical and basolateral membranes can also be measured. Newcells Biotech advises collecting information from three distinct biological donor kidneys of every species.
Source: Newcells Biotech
| Assay design |
| Models |
aProximate™ primary isolated kidney proximal tubule cells |
| Assay format |
24-well Transwell® plates (transporter assays) |
| 96-well Transwell® plates (kidney toxicity assays) |
| Species |
Human |
| Mouse |
| Rat |
| Dog |
| Service readouts |
Apical to Basal (Jab) and Basal to Apical (Jba) flux |
| Net transport measurements |
| Measurement of intracellular drug and metabolite concentrations |
| Kidney toxicity assays |
| Time points and replicates |
0, 30, 60, 90, 120 minutes (flux) |
| 72 hours (toxicity assays) |
| Data points are usually performed in triplicates |
Model available for this service
aProximate™ proximal tubule cells
aProximateTM is one of the most sophisticated, nearly physiological, in vitro kidney PTC models. Fresh human, rat, mouse, and kidney tissue cultured on Transwells® yields aProximate™ PTCs, which stay well differentiated as a polarized cell layer forming tight junctions.
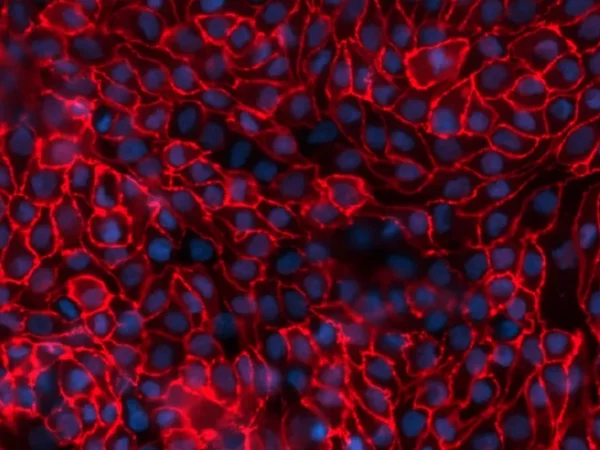
Image Credit: Newcells Biotech
Images
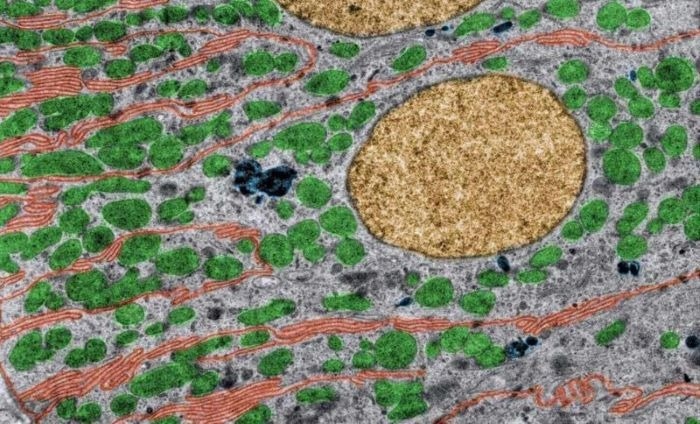
Colored Transmission electron microscopy (TEM) of kidney tubule cells. Basal infoldings are labeled in red color, mitochondria in green, nuclei in yellow, and lysosomes in dark blue. Kidney tubule cells contain many mitochondria to handle drugs and excrete xenobiotics. Mitochondrial health is a good indicator of overall cell heath and allows the evaluation of drug safety. Image Credit: Newcells Biotech
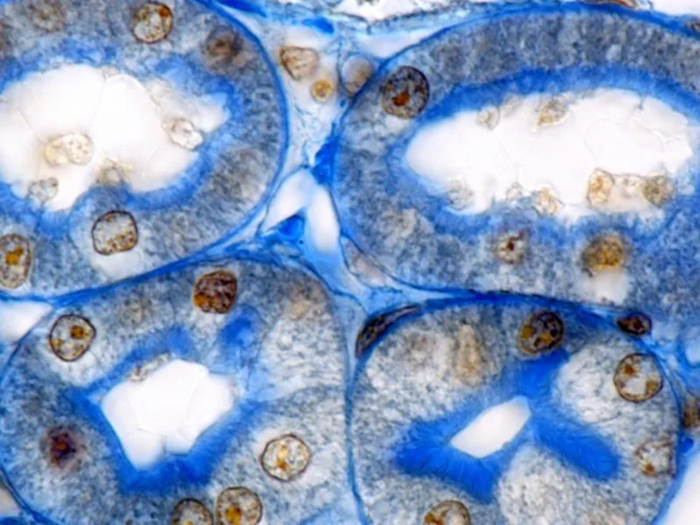
Kidney proximal tubules stained with Mallory's aniline. Both the brush border and the basement membrane surrounding the tubule are clearly highlighted. Image Credit: Newcells Biotech