Image Credit: Newcells Biotech
Drug-induced nephrotoxicity is one of the primary causes of drug failure, as predictions of kidney toxicity during new drug development are still inaccurate. Early detection is critical for reducing timelines and costs. The FDA recommends that new drugs and biologics be tested in vitro for nephrotoxicity before being tested in vivo in animal models.
Further regulatory guidance from the FDA and EMA recommends that early indicators of kidney toxicity, like KIM-1 and clusterin, which can only be measured in more intricate in vitro nephrotoxicity models, should also be detected. Such models are, therefore, required for renal safety profiles to be accurately predicted and evaluated early. The best method for determining renal toxicity is the Newcells aProximateTM proximal tubule cell model.
- Full safety profile
- Multiple species
- 96-well
Service outputs
- aProximateTM
- Evaluation of KIM-1, NGAL, and clusterin, three FDA-approved biomarkers of kidney toxicity
- Cell viability: ATP, LDH, and TEER measurements
Assays
- Kidney toxicity assays
- Assessment of renal drug safety
Models
- aProximateTM kidney proximal tubule cells (from human, mouse, rat, and dog)
Timeline
How can Newcells kidney tissue models be used to assess renal toxicity?
Newcells offers ProximateTM assay-ready plates for European and US customers to conduct renal toxicity tests in-house. Assay-ready plates are now available in 24- and 96-well Transwell® formats. The model comes with a maintenance medium and a user guide that includes a detailed protocol for cell recovery after receipt.
The consistent supply of fresh kidney tissue provided by Newcells allows for a quick and dependable in vitro nephrotoxicity service. Newcells Biotech collaborates to design experiments that address kidney safety and toxicity issues. The data will present key information about the compounds’ renal safety profiles, enabling efficient lead optimization. The custom projects are completed by professionals in cutting-edge UK facilities.
Example: In vitro nephrotoxicity screening
The model has been used to evaluate other compounds, and it correctly classified four of six true positives and two of three true negatives, demonstrating the validity of the in vitro nephrotoxicity model for detecting tubular toxicants. Quantifying translational biomarkers in freshly isolated aProximateTM PTCs can help assess nephrotoxicity in the drug discovery pipeline.
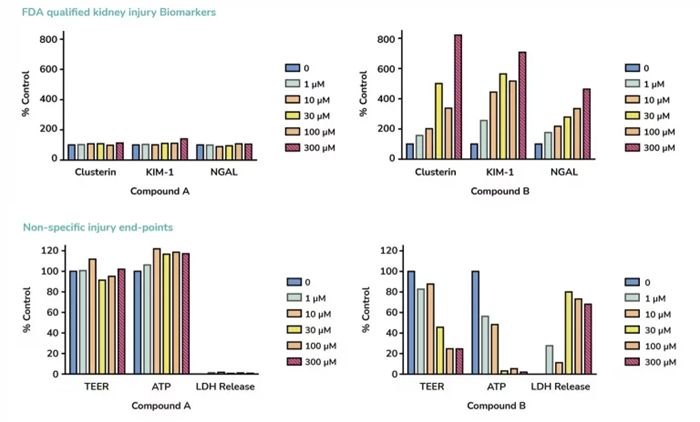
A high content comparison of two compounds for drug safety prediction using aProximateTM PTCs indicates a possible nephrotoxic effect for compound B with increased injury biomarkers and decreased non-specific injury endpoints. Compound A is predicted as non-toxic. Image Credit: Newcells Biotech
Service overview
Newcells Biotech offers insights into drug-induced nephrotoxicity.
Newcells evaluates compounds’ kidney toxicity in vitro using aProximateTM. In vitro proximal tubule nephrotoxicity testing will evaluate the compounds’ renal safety profiles, significantly accelerating drug safety studies.
Before additional in vivo animal testing, users can feel confident in the lead candidates thanks to the data produced by the renal toxicity assays, which offer a thorough understanding of the compounds' safety profile by using testing parameters that adhere to regulatory guidelines.
Additionally, aProximateTM enables the evaluation of nephrotoxicity in various pre-clinical species, including dogs, rats, and mice, to assist users in selecting the best in vivo model for the compound.
The consistent supply of fresh kidney tissue allows users to complete projects on short timelines. With the help of solid data produced by scientific experts, users can make confident decisions about important aspects of drug development.
Evaluating FDA-approved biomarkers (KIM-1, NGAL, and clusterin) in the aProximateTM proximal tubule cell model is an example of a renal toxicity package. The monolayer integrity and drug-induced cell health can be evaluated using both kidney tissue models. Three distinct biological donor kidneys from various species can be used to obtain data.
Source: Newcells Biotech
| Assay design |
| Models |
aProximateTM primary isolated kidney proximal tubule cells |
| Assay format |
96-well Transwell® plates |
| Species |
- Human (PTCs)
- Mouse (PTC)
- Rat (PTC)
- Dog (PTC)
|
| Assay readouts |
Nephrotoxicity assay
- Assessment of FDA-approved biomarkers of renal toxicity: KIM-1, NGAL and clusterin (ELISA/MSD)
- Cell viability: ATP, LDH, and TEER measurements
|
| Assay overview |
- 3 compounds, 6 concentrations per compound
- Time points: 0, 24 h up to 3 days
- Each data point performed in triplicates
|
Zo-1 Immunocytochemistry staining of PTCs (red) and Hoescht nuclear staining. Image Credit: Newcells Biotech
Model for this service
aProximateTM proximal tubule cells
aProximateTM is a highly advanced in vitro model of kidney PTCs that mimics physiological conditions. aProximateTM PTCs are derived from fresh human kidney tissue and grown on Transwells®. They remain well differentiated as a polarized cell layer with tight junctions.