Image Credit: Newcells Biotech
A robust model, such as aProximateTM, is required to study renal dysfunction and perform in vitro kidney disease modeling. This model expresses all transporters specialized in drug, solute, and protein handling. The Newcells platform is unique in that it accurately predicts in vivo outcomes.
It also provides insights into disease mechanisms and the evaluation of new drugs' therapeutic targets (for example, renal transporters). aProximateTM has been used to study various diseases, such as chronic kidney disease (CKD), gout, and metabolic disorders.
Service outputs
- aProximateTM PTC model
- Expression of renal transporters, high-throughput drug modulator screening for particular transporters, and Flux assays
Renal transporters disease modeling
- CKD: Calcium and phosphate transport imbalance
- Metabolic disorders: Amino acid transporter impairment
- Gout: Urate transporters deficiency
Models
- aProximateTM kidney proximal tubule cells (human, mouse, rat, and dog)
Species
How to use kidney models for renal disease modeling
Customers can use the freshly isolated proximal tubule cell kidney transporter model in their own lab to better understand possible cross-species differences in drug handling using Newcells’ Proximate™ human assay-ready plates, which are ready for shipment.
Thanks to the consistent supply of fresh kidney tissue, Newcells provides a quick and dependable service. Newcells Biotech designs and conducts the studies to answer drug-handling questions. The data will provide key insights into the compounds’ pharmacokinetic and ADME profiles, allowing for more efficient lead optimization. The data has been used in regulatory filings. A team of kidney experts works on bespoke projects in cutting-edge UK facilities.
Modelling molecular and cellular mechanisms of disease
The kidneys are essential organs that help maintain homeostasis and balance fluids, electrolytes, nutrients, and toxins. Renal dysfunction is caused by external factors such as drugs or toxins or associated with other underlying conditions affecting the pancreas and heart, such as diabetes and cardiovascular disease.
Rare hereditary metabolic disorders also impact vital organs like the kidney. Through a complex network of transporters expressed at the apical and basolateral cell membranes, the kidney's proximal tubule cells ensure waste is eliminated from the blood and nutrients are reabsorbed from the urine. Kidney function is impacted when this balance is upset.
Chronic Kidney Disease – calcium and phosphate transport balance
Any change in kidney structure or function that affects a person's health for more than three months, regardless of the cause, is called CKD. Dialysis and kidney transplantation are used to treat the end stages of CKD, while the early stages are asymptomatic.
Chronic inflammation, fibrosis, uremic toxins, vascular calcification, and an imbalance in the renin-angiotensin-aldosterone system are the main cellular and molecular processes that lead to CKD.
Diabetes mellitus and cardiovascular disease, two conditions linked to chronic kidney disease, also cause vascular calcification. Vascular calcification results from an imbalance in calcium and phosphate homeostasis, frequently caused by a reduction in renal phosphate clearance.
CKD treatment targets include renal sodium and phosphate co-transporters, such as sodium-phosphate transporters type I and II (NaPi-I and NaPi-II). Since aProximateTM expresses these and other transporters, it is a perfect tool for simulating CKD in vitro by determining the rate at which phosphate accumulates intracellularly.
Metabolic disorders – impairment of renal tubular transport of amino acids
The movement of solutes like salts and amino acids across the apical membrane of proximal tubule cells is impacted by hereditary diseases with inborn metabolic errors. This leads to excess urine excretion and amino acid loss from the body because it impairs the reabsorption of essential amino acids.
The most common disorders are caused by mutations in solute carrier membrane transport proteins (e.g., SLC3, SLC6, SLC7, and SLC15). These mutations are found in metabolic disorders like Iminoglycinuria, Cystinuria, Fanconi Syndrome, Nephrolithiasis, Hartnup Disorder, and Gitelman Syndrome.
aProximateTM proximal tubule cells express SLC family transporters (SLC3, SLC6, SLC7, SLC15), allowing for the measurement and modeling of essential amino acid uptake by PTCs. aProximateTM PTC models enable high-throughput screening of drug modulators, including amino acid transporters.
Gout – uric acid transport
The kidney transports and eliminates waste products, including uric acid. When produced in excess, the kidneys cannot excrete it quickly enough, causing it to accumulate in the blood (hyperuricemia). Gout is a condition for which there is currently no effective clinical treatment. Gout is distinguished by elevated uric acid levels in the blood, which can also indicate CKD.
It has been demonstrated that urea transporters, including NPT1, NPT4, OAT1, OAT2, OAT3, OAT4, URAT1, GLUT9, ABCG2, and PDZK1, contribute to the elevation of serum uric acid levels, which makes them promising targets for novel gout therapies. Focusing on urate transporters and researching urate-lowering drugs in vitro can help understand hyperuricemia and associated conditions.
aProximateTM PTCs express urate transporters, allowing for easy in vitro high throughput inhibitor analysis. aProximateTM is highly predictive of in vivo outcomes, making it an ideal in vitro tool for understanding uric acid transport and evaluating new gout treatments.
Therapeutic oligonucleotide and siRNA delivery to the proximal tubule
Megalin and Cubilin are important uptake transporters of siRNA and oligonucleotides in the proximal tubule. They also carry large molecules like albumin, insulin, and aminoglycosides. aProximateTM proximal tubule cells express functional levels of Megalin and Cubilin, providing a unique in vitro model to test siRNA uptake by PTCs.
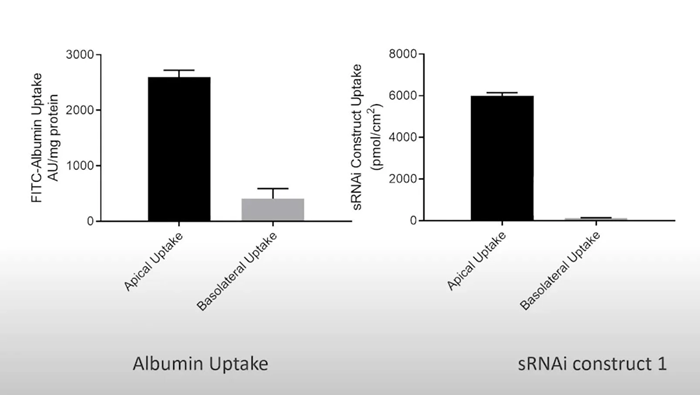
siRNA and albumin uptake experiments in freshly isolated primary proximal tubule cells. Image Credit: Newcells Biotech
Model for this service
aProximateTM proximal tubule cells
Image Credit: Newcells Biotech
aProximateTM is a cutting-edge in vitro model for kidney PTCs that mimics physiological conditions. aProximateTM PTCs are derived from fresh human, rat, mouse, and dog kidney tissue and grown on Transwells®. They form a polarized cell layer with tight junctions.