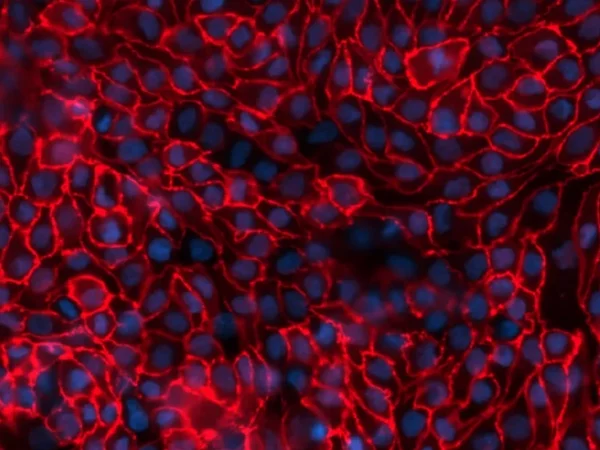
Tight junctions between aProximate™ proximal tubule cells shown by ZO-1 staining. Image Credit: Newcells Biotech
aProximateTM is an advanced in vitro model of kidney proximal tubule cells (PTCs) that closely mimics physiological conditions. Derived from fresh human kidney tissue, aProximateTM PTCs are cultured on Transwells® to form a functional, polarized cell layer with tight junctions.
Unlike other primary or immortalized kidney proximal tubule cells, aProximateTM PTCs maintain high expression of key transporters like Megalin and Cubilin, making them ideal for drug transporter studies.
Research using aProximateTM can provide a detailed understanding of how new drugs are transported and eliminated by the kidney and how they interact with other drugs, helping to assess and reduce the risk of renal toxicity.
Applications
- Nephrotoxicity assays
- Disease modeling
- Drug transporter interactions
- Drug-drug interactions (DDIs)
- Species comparison
Available analytical readouts
- The measurement of intracellular drug and metabolite concentrations
- Finding drug-drug interactions mediated by transporters
- Flux and net transport measurements
- Cell viability
- Early renal damage biomarkers
The model has been able to closely recapitulate human in vivo proximal tubule functionality and generated valuable insights regarding drug transport by primary human kidney cells. In my opinion, Newcells is the premiere provider of primary human renal cells with physiologically relevant functionality to support toxicity, renal clearance, and renal drug interaction screening.”
David Rodrigues, PhD, Senior Scientific Director, Pfizer
Model formats
- 24-well Transwells (static and flow)
- 96-well Transwells (static and flow)
- Assay-ready plates for shipment (Human, 24-well & 96-well)
Cell types
- aProximateTM - primary kidney proximal tubule cells
Species
How to use aProximateTM
Customers in the USA and Europe can order aProximateTM assay-ready plates from Newcells to conduct in-house kidney transporter and toxicity studies. Assay-ready plates are now offered in 24-well and 96-well Transwell® formats for both human and rat species. Upon receipt of the model, a maintenance medium and user guide containing a comprehensive protocol for cell recovery are provided.
Newcells' team works closely with clients to design experiments that deliver results within two months, offering fast and reliable service. Custom projects are completed using state-of-the-art facilities in the UK.
Tutorial: Revival of aProximate™ Assay Ready Plates
Revival of aProximate™ assay ready plates. Video Credit: Newcells Biotech
Applications for aProximateTM kidney proximal tubule cells
- Kidney toxicity assays
- Disease modeling
- Drug transporter interactions
- Drug-drug interactions
- Species comparison
Why use Newcells aProximateTM kidney proximal tubule cell model?
aProximateTM is the perfect model to use earlier and later in the drug development process to understand drug handling in the kidney's proximal tubule cells. Given that multiple drugs and metabolites are handled by the same transporters, in vitro drug testing and modeling in the proximal tubule is a popular method for reducing the risk of failure during preclinical and clinical development.
aProximate™ allows simple and accurate assessment of interactions between novel medications and renal transporters in vitro. The mechanistic insights offered can support regulatory applications or responses to regulatory agencies.
As evidenced by the loss of transporter and metabolic enzymes involved in drug transport and metabolism, primary kidney proximal tubule cells tend to dedifferentiate and lose important phenotypes over time in culture, following a freeze-thaw cycle and following immortalization.
They also exhibit different redox activity and a different equilibrium between glycolytic and non-glycolytic pathways than PTCs in vivo. As aProximateTM PTCs are newly isolated from kidney tissue, they maintain an accurate near-physiological phenotype and function, setting them apart from other PTC cells.
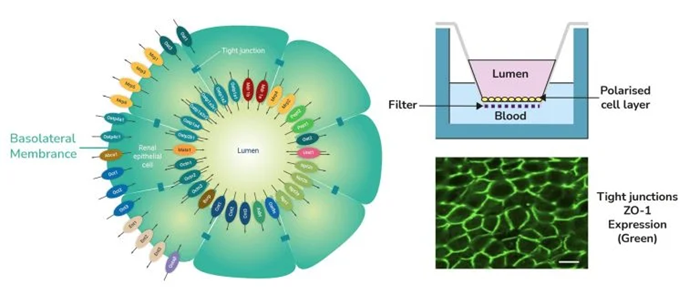
aProximate proximal tubule cell (PTC) model. Schematic diagram of aProximate PTCs showing the expression of all key renal transporters (left) and the formation of tight junctions as shown by ZO-1 tight junction protein labeling (bottom right). Diagram of Transwell plates demonstrating the aProximate model: PTCs grown on filters remain fully differentiated as a polarised cell layer (top right). Image Credit: Newcells Biotech
Nephrotoxicity assessment and prediction remain difficult parameters in drug development's early and late stages. Drugs like antibiotics, antiretrovirals, antisense oligonucleotides, and siRNA therapies, for instance, have been shown to build up in kidney proximal tubule cells, altering cell function and potentially causing nephrotoxicity.
To prevent drug-induced kidney damage during clinical trials, which is frequently missed in animal models because of their low sensitivity, reliable predictive or investigative toxicology tools for early evaluation are required.
More sensitive than blood and urine biomarkers like creatinine, aProximateTM enables the detection of FDA-qualified kidney-specific biomarkers in response to injury, such as KIM-1, NGAL, and Clusterin. This demonstrates that using aProximateTM as an in vitro model during drug discovery is appropriate for evaluating renal drug safety and kidney proximal tubule cell toxicity.
aProximateTM has been thoroughly validated for various applications.
aProximateTM PTC model description
- Layer of polarized cells with tight junctions
- Functional and fully differentiated kidney proximal tubule cells
- Expression of all important basolateral and apical transporters involved in drug handling, including Megalin and Cubilin
- Measurement of net flux
- Suitable for detection of clinical biomarkers for early nephrotoxicity
- Unique species comparison capability
- High throughput
aProximate™ expresses most relevant kidney transporters, unlike other primary and immortalized PTCs like RPTEC, KH2, and HEPTEC, which express very low levels (see table below).
Source: Newcells Biotech
| Transporter gene |
Relative mRNA expression of kidney transporters |
| Human aProximate™ PTC |
RPTEC |
| MDR1 |
65.2 ± 7.1 |
26 |
| BCRP |
31.3 ± 5.5 |
TBC |
| MRP1 |
31.5 ± 33 |
6 |
| MRP4 |
29.3 ± 4.8 |
24 |
| OAT1 |
20.6 ± 4.6 |
ND |
| OAT3 |
27.8 ± 6.7 |
ND |
| OCT2 |
39.7 ± 4.3 |
1.8 |
| OATP4C1 |
39.0 ± 2.7 |
34 |
| SLC2A9 |
27.7 ± 4.8 |
ND |
| URAT1 |
34.6 ± 9.2 |
ND |
| MATE1 |
36.4 ± 4.2 |
0.6 |
| MATE2K |
15.1 ± 8.8 |
0.3 |
aProximateTM assay-ready plates
Customers can now order aProximateTM for in-house studies on kidney transporters and toxicity. aProximateTM Assay-Ready plates (Human) are available in 24- or 96-well Transwell® format. The cells are shipped in stasis media and must be revived at 37 °C before use. The model has a maintenance medium and a user guide with a detailed recovery protocol.
Catalogue reference
Source: Newcells Biotech
| Model |
Sku no. |
Format |
Species |
Readouts |
| aProximateTM Assay-Ready Plates |
|
| Assay-Ready Plates |
KP0000HA24 |
24-Trans-wellsTM |
Human |
NA |
| Assay-Ready Plates |
KP0000RA24 |
24-Trans-wellsTM |
Human |
NA |
| Assay-Ready Plates |
KP0000HA96 |
96-Trans-wellsTM |
Human |
NA |
| Assay-Ready Plates |
KP0000RA24 |
96-Trans-wellsTM |
Human |
NA |
| Nephrotoxicity Assay |
|
| Nephrotoxicity |
KSN00000H |
96-Trans-wellsTM |
Human |
ELISA/MSD, TEER, ATP assay, LDH production |
| Nephrotoxicity |
KSN00000R |
96-Trans-wellsTM |
Rat |
ELISA/MSD, TEER, ATP assay, LDH production |
| Nephrotoxicity |
KSN00000M |
96-Trans-wellsTM |
Mouse |
ELISA/MSD, TEER, ATP assay, LDH production |
| Nephrotoxicity |
KSN00000D |
96-Trans-wellsTM |
Dog |
ELISA/MSD, TEER, ATP assay, LDH production |
| Drug Transporter Interactions & Drug-Drug Interactions |
|
| Drug Transporter Assay/ Drug Interactions/ Flux and Net Transporter Measurements/ Measurement of intracellular drug and metabolite concentrations |
KST00000H |
24-Trans-wellsTM |
Human |
Uptake/Flux measurement & imaging |
| Drug Transporter Assay/ Drug Interactions/ Flux and Net Transporter Measurements/ Measurement of intracellular drug and metabolite concentrations |
KST00000R |
24-Trans-wellsTM |
Rat |
Uptake/Flux measurement & imaging |
| Drug Transporter Assay/ Drug Interactions/ Flux and Net Transporter Measurements/ Measurement of intracellular drug and metabolite concentrations |
KST00000M |
24-Trans-wellsTM |
Mouse |
Uptake/Flux measurement & imaging |
| Drug Transporter Assay/ Drug Interactions/ Flux and Net Transporter Measurements/ Measurement of intracellular drug and metabolite concentrations |
KST00000D |
24-Trans-wellsTM |
Dog |
Uptake/Flux measurement & imaging |
| Disease Modelling |
|
| Calcium and Phosphate Transporters Imbalance/ Amino Acid Transporter Impairment/ Urate Transporters Deficiency |
KSD00000H |
24-Trans-wellsTM |
Human |
As per customer reqts. |
| Calcium and Phosphate Transporters Imbalance/ Amino Acid Transporter Impairment/Urate Transporters Deficiency |
KSD00000R |
24-Trans-wellsTM |
Rat |
As per customer reqts. |
Calcium and Phosphate Transporters Imbalance/ Amino Acid Transporter
Impairment/ Urate Transporters Deficiency |
KSD00000M |
24-Trans-wellsTM |
Mouse |
As per customer reqts. |
| Calcium and Phosphate Transporters Imbalance/ Amino Acid Transporter Impairment/ Urate Transporters Deficiency |
KSD00000D |
24-Trans-wellsTM |
Dog |
As per customer reqts. |
| Cross-Species Comparison |
|
| Drug transporter assays |
KST00HRMD |
24-Trans-wellsTM |
Human, Rat, Mouse, Dog |
Uptake/Flux measurement & imaging |
| Drug interactions |
KST00HRMD |
24-Trans-wellsTM |
Human, Rat, Mouse, Dog |
Uptake/Flux measurement & imaging |
| Flux and net flux drug transport |
KST00HRMD |
24-Trans-wellsTM |
Human, Rat, Mouse, Dog |
Uptake/Flux measurement & imaging |
| Intracellular drug and metabolite concentrations |
KST00HRMD |
24-Trans-wellsTM |
Human, Rat, Mouse, Dog |
Uptake/Flux measurement & imaging |
| Nephrotoxicity assays |
KSN00HRMD |
96-Trans-wellsTM |
Human, Rat, Mouse, Dog |
ELISA/MSD, TEER, ATP assay, LDH production |
| Renal drug safety evaluation |
KSN00HRMD |
96-Trans-wellsTM |
Human, Rat, Mouse, Dog |
TEER, ATP assay, FITC-Dextran Permeability, Imaging |
Images
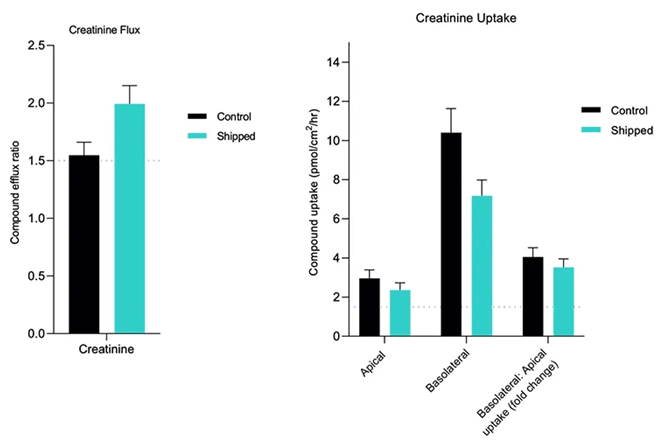
Functional validation of aProximateTM assay-ready plates for transporter assays Functional validation studies for transcellular flux and uptake of creatinine revealed preferential basolateral-to-apical (JBA) creatinine transport, indicative of normal physiological function. A JBA uptake ratio >1.5 indicates that the cells are functional. Image Credit: Newcells Biotech