Access to a steady supply of N95 respirator masks is lacking amid the ongoing SARS-CoV-2 outbreak, despite efforts by the private sector and global governments.
The irradiation and reuse of existing N95 masks is a vital mitigation approach to the limited supply of N95 masks, the CDC contends that it is a promising strategy, particularly during a pandemic1. A hospital-approved ultraviolet germicidal irradiation (UVGI) workflow for masks has become available recently2,3.
Healthcare workers (HCWs) should ideally not routinely resort to reusing masks. Yet, if required, the integration of the UVC-based CL-3000 crosslinker into a hospital-approved UVGI workflow will produce 1J/cm2 of UVC with reproducible results.
Background
By acting directly on the DNA/RNA of microorganisms, shortwave ultraviolet light (UVC) possesses germicidal properties. DNA/RNA absorbs ultraviolet light maximally at around 260 nm, which in turn, damages the DNA/RNA structure.
Even though microorganisms have mechanisms that can counteract this damage, they cannot overcome extensive high-intensity UV doses, which ultimately kill or inactivate the microorganism.
In the scientific literature for a range of viruses4–37‡, ultraviolet germicidal irradiation (UVGI) of viruses has been seen extensively, and most recently for virus-contaminated N95 filtering facepiece respirators (FFRs)2,3,7,27,34‡‡.
The University of Nebraska Medical Center (UNMC) and Stanford University have developed UVGI workflows to be implemented by HCWs2,3 in order to prepare for inevitable shortages of N95 FFRs during the ongoing SARS-CoV-2 pandemic.
These workflows take advantage of UV equipment which is sometimes already found in the healthcare environment. These include, but are not limited to, whole-room UV emitters2,15 and UV disinfection boxes.3
Some key concerns regarding these instruments are the risk of inconsistent dosing between disinfection cycles, the lack of uniformity in germicidal light, plus the risk of UV exposure to HCWs in general.38,39
Analytik Jena believes that they have a better solution. They have been supplying the UVP Crosslinker (CL-1000/CX-2000) since 1993, mainly for molecular biology applications, which are reliant on reproducible, high-intensity UVC doses.
They can achieve highly uniform illumination (Figure 1B and 1C) by having reflective housing and the UV source in close proximity to the sample (Figure 1A). The CL-3000 (Figure 1A), which is the most recent model, is designed with a built-in radiometer that is calibrated to a NIST traceable standard. This ensures consistent doses irrespective of time and space.
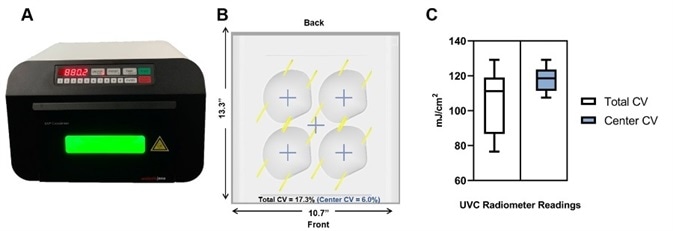
Figure 1. Uniformity of the Analytik Jena UVP Crosslinker equipped with 254 nm UVC bulbs. A) Profile image of the UVP CL-3000 Crosslinker during a disinfection cycle. B) Diagram depicting the position of integrated radiometer readings in mJ/cm2 with dimensions and calculated coefficient of variation (CV) for the entire illumination surface (Total CV), and the center of the illumination surface (Center CV), the latter of which is where masks should be placed. C) Box-whisker plot summary of radiometer readings. Total CV: Mean =104.9, SD= 18.16; Center CV: Mean = 117.9, SD=7.1. We recommend users place masks in the center of the illumination to maximize the evenness of UV exposure. Samples place in the center will receive, at a minimum, the input energy dose. If users want to place samples at the front-most and back-most positions, we recommend purchasing a UVP 254 nm radiometer (P/N 97-0015-02) and sensor (P/N 97-0016-01) to calibrate dose.
Furthermore, the CL-3000 can generate a cumulative dose of up to 10J/cm2. The safety of the end-user is critical, as with all of their instruments, so all of the Crosslinkers possess a safety-interlock to prevent accidental UV exposure.
Crucially, there is an extensive body of scientific work where their Crosslinker is utilized for viral inactivation of viruses from several families11,18,19,24–26,31–33,35,36, including coronaviruses21,23,30, which is lacking for other instruments on the market.
Analytik Jena agrees with the CDC, which contends that UVGI is a viable crisis strategy for the capacity management of personal protective equipment1. In summary, their Crosslinker can be integrated easily into UVGI mask disinfection workflows for the following reasons:
- Proven history of performance from the scientific literature
- Embedded radiometer calibrated to NIST traceable standards to ensure reproducible dosing
- Safety interlock to prevent accidental exposure
- Fixed distance between sample and UVC source to ensure uniform dosing
- Small footprint to accommodate limited spaces and/or decentralization
- Closed system to accommodate scaling up strategies
- Higher UVC output for shorter disinfection cycles
Operating the CL-3000 as part of an approved UVGI workflow
As stated in an FDA commissioned a report, a dose of 1J/cm2 is enough to result in no detectable virus on N95 mask material after UVGI treatment, corresponding to a 3.9-4.5 log10 reduction for MERS-CoV and 4.0-4.8 log10 reduction for SARS-CoV, between experimental and control groups34.
How to utilize the device to achieve the same irradiation level of 1J/cm2 is described below. After each disinfection cycle, HCWs should inspect masks for wear and tear.2,3,13,34
How to operate the CL-3000
- Set the dosage on the instrument by selecting ENERGY, then pressing 1-0-0-0-0 for 1000.0 mJ/cm2 (or 1J/cm2), and then press ENTER.
- Place the mask into the center-most portion of the chamber and close the door.
- Press START.
- Open the door, flip the mask over, and repeat step 3.
- Remove the sample and continue following the UVGI protocol approved by your institution or hospital.
Note: As an added precaution, users may consider repeating steps 1-3 with an empty crosslinker in between disinfection cycles if residual contamination is a concern.
Disclaimer: Analytik Jena does not advocate specific treatments or approaches. Analytik Jena is simply sharing the most recent evidence from the medical community to help HCWs during the SARS-CoV-2 pandemic. Your UVGI workflow should be set and approved by your hospital/institution. Users can refer to the UNMC2 and/or Stanford University3 workflows for process recommendations.
Technical data
Table 1.
| Technical Specifications |
CL-3000 |
| Wavelength |
254 nm |
| Bulbs |
6 x 8 Watt |
| Energy |
0000.1 - 9999.9 mJ/cm² (0 - 10 J/cm²) |
| Time |
000:01 - 999:59 mmm:ss (>300J/cm²) |
| Temperature |
15°C - 35°C |
| Humidity |
70% Non-Condensing |
| Altitude |
up to 3,000M (9,842 ft) |
| Sound Level |
≤ 50 dba |
| Housing Surface Temp |
≤ 30°C |
| Startup Time |
< 1 sec |
| External Dim (L x W x H) |
41cm x 40cm x 26.5cm |
| Internal Dim (L x W x H) |
35cm x 27cm x 16cm |
| Weight |
6.8Kg: 15 lb |
| Operating Power |
100 - 115VAC & 230VAC 50/60Hz |
| Certifications |
CE, RoHS (CSA In Process) |
Part numbers and description
Table 2.
| Part Number |
Description |
| 849-95-0615-01 |
UVP Crosslinker (CL-3000), 254 nm, 100 – 115V |
|
| 849-95-0615-02 |
UVP Crosslinker (CL-3000), 254 nm, 230V |
Mid-Range Wavelength: 302 nm (CL-3000M) and Long-Range Wavelength: 365 nm (CL-3000L) UVP Crosslinkers are available to order but are not applicable to this application note.
References and Further Reading
- CDC. Coronavirus Disease 2019 (COVID-19) - Decontamination and Reuse of filtering Facepiece Respirators. Centers for Disease Control and Prevention https://www.cdc.gov/ (2020).
- CLowe, J. et al. N95 Filtering Facemask Respirator Ultraviolet Germicidal Irradiation (UVGI) Process for Decontamination and Reuse. https://www.nebraskamed.com/sites/default/files/documents/covid-19/n-95-decon-process.pdf (2020).
- Price, A. & Chu, L. COVID-19 Evidence Service - Addressing COVI-19 Face Mask Shortages [v1.2]. https://www.iapb.org/wp-content/uploads/2020/11/mask-ppe-EBM-v1.2-3-25-2020.pdf.
- Darnell, M. E. R., Subbarao, K., Feinstone, S. M. & Taylor, D. R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. Journal of Virological Methods 121, 85–91 (2004).
- McDevitt, J. J., Milton, D. K., Rudnick, S. N. & First, M. W. Inactivation of Poxviruses by Upper-Room UVC Light in a Simulated Hospital Room Environment. PLoS ONE 3, e3186 (2008).
- Rutala, W. A., Gergen, M. F. & Weber, D. J. Room Decontamination with UV Radiation. Infect. Control Hosp. Epidemiol. 31, 1025–1029 (2010).
- Fisher, E. M. & Shaffer, R. E. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. Journal of Applied Microbiology 110, 287–295 (2011).
- Park, G. w., Linden, K. g. & Sobsey, M. d. Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Letters in Applied Microbiology 52, 162–167 (2011).
- Zou, S. et al. Inactivation of the novel avian influenza A (H7N9) virus under physical conditions or chemical agents treatment. Virol J 10, 289 (2013).
- Kiryu, I., Sakai, T., Kurita, J. & Iida, T. Virucidal Effect of Disinfectants on Spring Viremia of Carp Virus. Fish Pathol. 42, 111–113 (2007).
- Singh, R., Sharma, A., Hong, S. & Jang, J. Electrical immunosensor based on dielectrophoretically-deposited carbon nanotubes for detection of influenza virus H1N1. Analyst 139, 5415–5421 (2014).
- Beck, S. E., Wright, H. B., Hargy, T. M., Larason, T. C. & Linden, K. G. Action spectra for validation of pathogen disinfection in medium-pressure ultraviolet (UV) systems. Water Research 70, 27–37 (2015).
- Lindsley, W. G. et al. Effects of Ultraviolet Germicidal Irradiation (UVGI) on N95 Respirator Filtration Performance and Structural Integrity. Journal of Occupational and Environmental Hygiene 12, 509–517 (2015).
- Bae, K. S., Shin, G.-A., Bae, K. S. & Shin, G.-A. Inactivation of various bacteriophages by different ultraviolet technologies: Development of a reliable virus indicator system for water reuse. Environmental Engineering Research 21, 350–354 (2016).
- Bedell, K., Buchaklian, A. & Perlman, S. Efficacy of an automated multi-emitter whole room UV-C disinfection system against Coronaviruses MHV and MERS-CoV. Infect Control Hosp Epidemiol 37, 598–599 (2016).
- Chu, H.-A. & Chiu, Y.-F. The Roles of Cytochrome b559 in Assembly and Photoprotection of Photosystem II Revealed by Site-Directed Mutagenesis Studies. Front. Plant Sci. 6, (2016).
- Song, K., Mohseni, M. & Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Research 94, 341–349 (2016).
- Ziegler, C. M. et al. The Lymphocytic Choriomeningitis Virus Matrix Protein PPXY Late Domain Drives the Production of Defective Interfering Particles. PLoS Pathog 12, (2016).
- Berger, A. K. et al. Viral RNA at Two Stages of Reovirus Infection Is Required for the Induction of Necroptosis. J. Virol. 91, e02404-16, /jvi/91/6/e02404-16.atom (2017).
- Fryk, J. J. et al. Reduction of Zika virus infectivity in platelet concentrates after treatment with ultraviolet C light and in plasma after treatment with methylene blue and visible light. Transfusion 57, 2677–2682 (2017).
- Fung, T. S. & Liu, D. X. Activation of the c-Jun NH 2 -terminal kinase pathway by coronavirus infectious bronchitis virus promotes apoptosis independently of c-Jun. Cell Death & Disease 8, 1–13 (2017).
- Kim, D.-K., Kim, S.-J. & Kang, D.-H. Inactivation modeling of human enteric virus surrogates, MS2, Qβ, and ΦX174, in water using UVC-LEDs, a novel disinfecting system. Food Research International 91, 115–123 (2017).
- Bodmer, B. S., Fiedler, A. H., Hanauer, J. R. H., Prüfer, S. & Mühlebach, M. D. Live-attenuated bivalent measles virusderived vaccines targeting Middle East respiratory syndrome coronavirus induce robust and multifunctional T cell responses against both viruses in an appropriate mouse model. Virology 521, 99–107 (2018).
- Frascaroli, G. et al. Human Macrophages Escape Inhibition of Major Histocompatibility Complex-Dependent Antigen Presentation by Cytomegalovirus and Drive Proliferation and Activation of Memory CD4+ and CD8+ T Cells. Front. Immunol. 9, (2018).
- Lee, M. K. et al. Transmembrane Protein pUL50 of Human Cytomegalovirus Inhibits ISGylation by Downregulating UBE1L. J Virol 92, e00462-18, /jvi/92/15/e00462-18.atom (2018).
- Mathew, A. M., Mun, A. B. & Balakrishnan, A. Ultraviolet Inactivation of Chikungunya Virus. Intervirology 61, 36–41 (2018).
- Mills, D., Harnish, D. A., Lawrence, C., Sandoval-Powers, M. & Heimbuch, B. K. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. American Journal of Infection Control 46, e49–e55 (2018).
- Nishisaka-Nonaka, R. et al. Irradiation by ultraviolet light-emitting diodes inactivates influenza viruses by inhibiting replication and transcription of viral RNA in host cells. Journal of Photochemistry and Photobiology B: Biology 189, 193–200 (2018).
- Vaidya, V. et al. Ultraviolet-C irradiation for inactivation of viruses in foetal bovine serum. Vaccine 36, 4215–4221 (2018).
- Yang, L. et al. Porcine Epidemic Diarrhea Virus-Induced Epidermal Growth Factor Receptor Activation Impairs the Antiviral Activity of Type I Interferon. J Virol 92, e02095-17, /jvi/92/8/e02095-17.atom (2018).
- Baidaliuk, A. et al. Cell-Fusing Agent Virus Reduces Arbovirus Dissemination in Aedes aegypti Mosquitoes In Vivo. J Virol 93, e00705-19, /jvi/93/18/JVI.00705-19.atom (2019).
- Campbell, T. M. et al. Functional paralysis of human natural killer cells by alphaherpesviruses. PLoS Pathog 15, (2019).
- DeFord, D. M. et al. Evaluation of the role of respiratory syncytial virus surface glycoproteins F and G on viral stability and replication: implications for future vaccine design. Journal of General Virology, 100, 1112–1122 (2019).
- Heimbuch, B. & Harnish, D. Research to mitigate a shortage of respiratory protection devices during public health emergencies. (2019).
- Lee, A. C. Y. et al. H7N9 influenza A virus activation of necroptosis in human monocytes links innate and adaptive immune responses. Cell Death & Disease 10, 1–16 (2019).
- Romero, N., Van Waesberghe, C. & Favoreel, H. W. Pseudorabies virus infection of epithelial cells leads to persistent but aberrant activation of the NF-κB pathway, inhibiting hallmark NF-κB-induced pro-inflammatory gene expression. J Virol JVI.00196-20, jvi;JVI.00196-20v1 (2020) doi:10.1128/JVI.00196-20.
- Zhao, X. et al. Activation of C-Type Lectin Receptor and (RIG)-I-Like Receptors Contributes to Proinflammatory Response in Middle East Respiratory Syndrome Coronavirus-Infected Macrophages. J Infect Dis 221, 647–659 (2020).
- Zaffina, S. et al. Accidental exposure to UV radiation produced by germicidal lamp: case report and risk assessment. Photochem. Photobiol. 88, 1001–1004 (2012).
- International Commission on Non-Ionizing Radiation Protection. ICNIRP statement-Protection of workers against ultraviolet radiation. Health Phys 99, 66–87 (2010).
‡These citations do not represent the entire body of literature for UVGI of viruses and only serve to represent the diversity of viruses that have been inactivated with UVGI.
‡‡ As of 4/7/2020 Ref. 2 & 3 have only been published online and are not peer-reviewed.
About Analytik Jena US
Analytik Jena is a provider of instruments and products in the areas of analytical measuring technology and life science. Its portfolio includes the most modern analytical technology and complete systems for bioanalytical applications in the life science area.
Comprehensive laboratory software management and information systems (LIMS), service offerings, as well as device-specific consumables and disposables, such as reagents or plastic articles, complete the Group’s extensive range of products.
About life science
The Life Science product area demonstrates the biotechnological competence of Analytik Jena AG. We provide a wide product spectrum for automated total, as well as individual solutions for molecular diagnostics. Our products are focused to offer you a quality and the reproducibility of your laboratory results.
This will surely ease your daily work and speed up your work processes in a certain way. All together we support you through the complete process of the lab work. Besides we offer customized solutions and are able to adapt our products to your needs. Automated high-throughput screening systems for the pharmaceutical sector are also part of this segment’s extensive portfolio.
About analytical instrumentation
Analytik Jena has a long tradition in developing high-performance precision analytical systems which dates back to the inventions made by Ernst Abbe and Carl Zeiss. We have grown to become one of the most innovative manufacturers of analytical measuring technology worldwide.
Our business unit Analytical Instrumentation offers excellent competencies in the fields of optical spectroscopy, sum parameters and elemental analysis. Being proud of our core competency we grant all our customers a long-term warranty of 10 years for our high-performance optics.
About lab automation
With more than 25 years of market experience, Analytik Jena with its CyBio® Product Line is a leading provider for high quality liquid handling and automation technologies. In the pharmaceutical and life science industries, our products enjoy the highest reputation for precision, reliability, robustness and simplicity.
Moreover, the Automation Team designs, produces and installs fully automated systems tailored to our clients' application, throughput and capacity requirements. From stand-alone CyBio® Well up to fully customized robotic systems we handle your compounds, biomolecules and cells with great care.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.